Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities
- PMID: 21724987
- PMCID: PMC3141932
- DOI: 10.1073/pnas.1019434108
Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities
Abstract
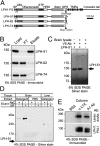
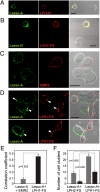
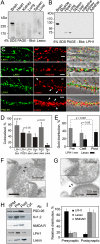
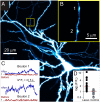
Latrophilin 1 (LPH1), a neuronal receptor of α-latrotoxin, is implicated in neurotransmitter release and control of presynaptic Ca(2+). As an "adhesion G-protein-coupled receptor," LPH1 can convert cell surface interactions into intracellular signaling. To examine the physiological functions of LPH1, we used LPH1's extracellular domain to purify its endogenous ligand. A single protein of ∼275 kDa was isolated from rat brain and termed Lasso. Peptide sequencing and molecular cloning have shown that Lasso is a splice variant of teneurin-2, a brain-specific orphan cell surface receptor with a function in neuronal pathfinding and synaptogenesis. We show that LPH1 and Lasso interact strongly and specifically. They are always copurified from rat brain extracts. Coculturing cells expressing LPH1 with cells expressing Lasso leads to their mutual attraction and formation of multiple junctions to which both proteins are recruited. Cells expressing LPH1 form chimerical synapses with hippocampal neurons in cocultures; LPH1 and postsynaptic neuronal protein PSD-95 accumulate on opposite sides of these structures. Immunoblotting and immunoelectron microscopy of purified synapses and immunostaining of cultured hippocampal neurons show that LPH1 and Lasso are enriched in synapses; in both systems, LPH1 is presynaptic, whereas Lasso is postsynaptic. A C-terminal fragment of Lasso interacts with LPH1 and induces Ca(2+) signals in presynaptic boutons of hippocampal neurons and in neuroblastoma cells expressing LPH1. Thus, LPH1 and Lasso can form transsynaptic complexes capable of inducing presynaptic Ca(2+) signals, which might affect synaptic functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA. Isolation and biochemical characterization of a Ca2+-independent α-latrotoxin–binding protein. J Biol Chem. 1996;271:23239–23245. - PubMed
-
- Lelianova VG, et al. The α-latrotoxin receptor latrophilin is a novel member of the secretin family of G protein–coupled receptors. J Biol Chem. 1997;272:21504–21508. - PubMed
-
- Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: Multiply spliced G protein–coupled receptors with differential tissue distribution. FEBS Lett. 1999;443:348–352. - PubMed
-
- Volynski KE, et al. Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation: This action does not require neurexins. J Biol Chem. 2003;278:31058–31066. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BB/D523078/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT083199MF/WT_/Wellcome Trust/United Kingdom
- BBC5196701/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBF0083091/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0600089/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

