Methyl fluoride affects methanogenesis rather than community composition of methanogenic archaea in a rice field soil
- PMID: 23341965
- PMCID: PMC3544908
- DOI: 10.1371/journal.pone.0053656
Methyl fluoride affects methanogenesis rather than community composition of methanogenic archaea in a rice field soil
Abstract
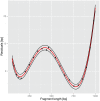
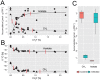
The metabolic pathways of methane formation vary with environmental conditions, but whether this can also be linked to changes in the active archaeal community structure remains uncertain. Here, we show that the suppression of aceticlastic methanogenesis by methyl fluoride (CH(3)F) caused surprisingly little differences in community composition of active methanogenic archaea from a rice field soil. By measuring the natural abundances of carbon isotopes we found that the effective dose for a 90% inhibition of aceticlastic methanogenesis in anoxic paddy soil incubations was <0.75% CH(3)F (v/v). The construction of clone libraries as well as t-RFLP analysis revealed that the active community, as indicated by mcrA transcripts (encoding the α subunit of methyl-coenzyme M reductase, a key enzyme for methanogenesis), remained stable over a wide range of CH(3)F concentrations and represented only a subset of the methanogenic community. More precisely, Methanocellaceae were of minor importance, but Methanosarcinaceae dominated the active population, even when CH(3)F inhibition only allowed for aceticlastic methanogenesis. In addition, we detected mcrA gene fragments of a so far unrecognised phylogenetic cluster. Transcription of this phylotype at methyl fluoride concentrations suppressing aceticlastic methanogenesis suggests that the respective organisms perform hydrogenotrophic methanogenesis. Hence, the application of CH(3)F combined with transcript analysis is not only a useful tool to measure and assign in situ acetate usage, but also to explore substrate usage by as yet uncultivated methanogens.
Conflict of interest statement
Figures


References
-
- Conrad R, Frenzel P (2002) Flooded soils. In: Britton G, editor. New York: John Wiley & Sons. pp. 1316–1333.
-
- Conrad R, Klose M (1999) How specific is the inhibition by methyl fluoride of acetoclastic methanogenesis in anoxic rice field soil? FEMS Microbiol Ecol 30: 47–56.
-
- Oremland RS, Capone DG (1988) Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol 10: 285–383.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

