Piezo proteins are pore-forming subunits of mechanically activated channels
- PMID: 22343900
- PMCID: PMC3297710
- DOI: 10.1038/nature10812
Piezo proteins are pore-forming subunits of mechanically activated channels
Abstract
Mechanotransduction has an important role in physiology. Biological processes including sensing touch and sound waves require as-yet-unidentified cation channels that detect pressure. Mouse Piezo1 (MmPiezo1) and MmPiezo2 (also called Fam38a and Fam38b, respectively) induce mechanically activated cationic currents in cells; however, it is unknown whether Piezo proteins are pore-forming ion channels or modulate ion channels. Here we show that Drosophila melanogaster Piezo (DmPiezo, also called CG8486) also induces mechanically activated currents in cells, but through channels with remarkably distinct pore properties including sensitivity to the pore blocker ruthenium red and single channel conductances. MmPiezo1 assembles as a ∼1.2-million-dalton homo-oligomer, with no evidence of other proteins in this complex. Purified MmPiezo1 reconstituted into asymmetric lipid bilayers and liposomes forms ruthenium-red-sensitive ion channels. These data demonstrate that Piezo proteins are an evolutionarily conserved ion channel family involved in mechanotransduction.
Figures


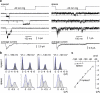
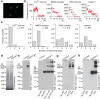
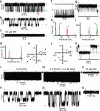
Comment in
-
Cell biology: The sensation of stretch.Nature. 2012 Mar 7;483(7388):163-4. doi: 10.1038/483163a. Nature. 2012. PMID: 22398551 Free PMC article.
References
-
- McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett. 1999;273:179–182. - PubMed
-
- Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992;262:C1083–1088. - PubMed
-
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. - PubMed
-
- Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

