Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin
- PMID: 22366317
- PMCID: PMC3311370
- DOI: 10.1073/pnas.1115485109
Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin
Abstract
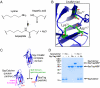
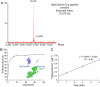
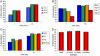
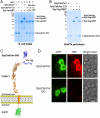
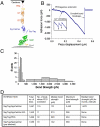
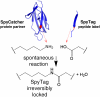
Protein interactions with peptides generally have low thermodynamic and mechanical stability. Streptococcus pyogenes fibronectin-binding protein FbaB contains a domain with a spontaneous isopeptide bond between Lys and Asp. By splitting this domain and rational engineering of the fragments, we obtained a peptide (SpyTag) which formed an amide bond to its protein partner (SpyCatcher) in minutes. Reaction occurred in high yield simply upon mixing and amidst diverse conditions of pH, temperature, and buffer. SpyTag could be fused at either terminus or internally and reacted specifically at the mammalian cell surface. Peptide binding was not reversed by boiling or competing peptide. Single-molecule dynamic force spectroscopy showed that SpyTag did not separate from SpyCatcher until the force exceeded 1 nN, where covalent bonds snap. The robust reaction conditions and irreversible linkage of SpyTag shed light on spontaneous isopeptide bond formation and should provide a targetable lock in cells and a stable module for new protein architectures.
Conflict of interest statement
Conflict of interest statement: M.H. and B.Z. are authors on a patent application regarding peptide targeting via spontaneous amide bond formation (United Kingdom Patent Application No. 1002362.0).
Figures
References
-
- Jarvik JW, Telmer CA. Epitope tagging. Annu Rev Genet. 1998;32:601–618. - PubMed
-
- Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. - PubMed
-
- Allen KN, Imperiali B. Lanthanide-tagged proteins--an illuminating partnership. Curr Opin Chem Biol. 2010;14:247–254. - PubMed
-
- Huh WK, et al. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

