Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae)
- PMID: 22700981
- PMCID: PMC3411041
- DOI: 10.1074/jbc.M112.370841
Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae)
Abstract
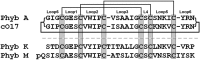
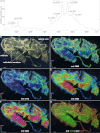
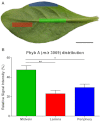
Cyclotides are a large family of plant peptides that are structurally defined by their cyclic backbone and a trifecta of disulfide bonds, collectively known as the cyclic cystine knot (CCK) motif. Structurally similar cyclotides have been isolated from plants within the Rubiaceae, Violaceae, and Fabaceae families and share the CCK motif with trypsin-inhibitory knottins from a plant in the Cucurbitaceae family. Cyclotides have previously been reported to be encoded by dedicated genes or as a domain within a knottin-encoding PA1-albumin-like gene. Here we report the discovery of cyclotides and related non-cyclic peptides we called "acyclotides" from petunia of the agronomically important Solanaceae plant family. Transcripts for petunia cyclotides and acyclotides encode the shortest known cyclotide precursors. Despite having a different precursor structure, their sequences suggest that petunia cyclotides mature via the same biosynthetic route as other cyclotides. We assessed the spatial distribution of cyclotides within a petunia leaf section by MALDI imaging and observed that the major cyclotide component Phyb A was non-uniformly distributed. Dissected leaf midvein extracts contained significantly higher concentrations of this cyclotide compared with the lamina and outer margins of leaves. This is the third distinct type of cyclotide precursor, and Solanaceae is the fourth phylogenetically disparate plant family to produce these structurally conserved cyclopeptides, suggesting either convergent evolution upon the CCK structure or movement of cyclotide-encoding sequences within the plant kingdom.
Figures



References
-
- Craik D. J., Daly N. L., Bond T., Waine C. (1999) Plant cyclotides. A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 294, 1327–1336 - PubMed
-
- Plan M. R., Saska I., Cagauan A. G., Craik D. J. (2008) Backbone cyclized peptides from plants show molluscicidal activity against the rice pest Pomacea canaliculata (golden apple snail). J. Agric. Food Chem. 56, 5237–5241 - PubMed
-
- Colgrave M. L., Kotze A. C., Huang Y. H., O'Grady J., Simonsen S. M., Craik D. J. (2008) Cyclotides. Natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry 47, 5581–5589 - PubMed
-
- Gran L. (1973) On the effect of a polypeptide isolated from “Kalata-Kalata” (Oldenlandia affinis DC) on the estrogen-dominated uterus. Acta Pharmacol. Toxicol. 33, 400–408 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

