Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria
- PMID: 23408630
- PMCID: PMC3624331
- DOI: 10.1128/JVI.02954-12
Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria
Abstract
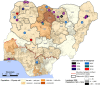
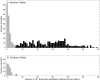
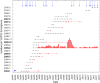
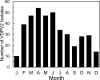
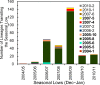
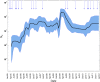
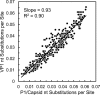
Since 2005, a large poliomyelitis outbreak associated with type 2 circulating vaccine-derived poliovirus (cVDPV2) has occurred in northern Nigeria, where immunization coverage with trivalent oral poliovirus vaccine (tOPV) has been low. Phylogenetic analysis of P1/capsid region sequences of isolates from each of the 403 cases reported in 2005 to 2011 resolved the outbreak into 23 independent type 2 vaccine-derived poliovirus (VDPV2) emergences, at least 7 of which established circulating lineage groups. Virus from one emergence (lineage group 2005-8; 361 isolates) was estimated to have circulated for over 6 years. The population of the major cVDPV2 lineage group expanded rapidly in early 2009, fell sharply after two tOPV rounds in mid-2009, and gradually expanded again through 2011. The two major determinants of attenuation of the Sabin 2 oral poliovirus vaccine strain (A481 in the 5'-untranslated region [5'-UTR] and VP1-Ile143) had been replaced in all VDPV2 isolates; most A481 5'-UTR replacements occurred by recombination with other enteroviruses. cVDPV2 isolates representing different lineage groups had biological properties indistinguishable from those of wild polioviruses, including efficient growth in neuron-derived HEK293 cells, the capacity to cause paralytic disease in both humans and PVR-Tg21 transgenic mice, loss of the temperature-sensitive phenotype, and the capacity for sustained person-to-person transmission. We estimate from the poliomyelitis case count and the paralytic case-to-infection ratio for type 2 wild poliovirus infections that ∼700,000 cVDPV2 infections have occurred during the outbreak. The detection of multiple concurrent cVDPV2 outbreaks in northern Nigeria highlights the risks of cVDPV emergence accompanying tOPV use at low rates of coverage in developing countries.
Figures

References
-
- Centers for Disease Control and Prevention 2001. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb. Mortal. Wkly. Rep. 50:222–224 - PubMed
-
- World Health Organization 2012. Progress towards global interruption of wild poliovirus transmission, January 2011-March 2012. Wkly. Epidemiol. Rec. 87:195–200 - PubMed
-
- Sutter RW, Kew OM, Cochi SL, Aylward RB. 2013. Poliovirus vaccine—live, p 598–645 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed WB Saunders Company, Philadelphia, PA
-
- Benyesh-Melnick M, Melnick JL, Rawls WE, Wimberley I, Barrera-Oro J, Ben-Porath E, Rennick V. 1967. Studies on the immunogenicity, communicability, and genetic stability of oral poliovaccine administered during the winter. Am. J. Epidemiol. 86:112–136 - PubMed
-
- Chen RT, Hausinger S, Dajani AS, Hanfling M, Baughman AL, Pallansch MA, Patriarca PA. 1996. Seroprevalence of antibody against poliovirus in inner-city preschool children. Implications for vaccination policy in the United States. JAMA 275:1639–1645 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

