Oral direct thrombin inhibitor AZD0837 for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: a randomized dose-guiding, safety, and tolerability study of four doses of AZD0837 vs. vitamin K antagonists
- PMID: 19690349
- PMCID: PMC2785945
- DOI: 10.1093/eurheartj/ehp318
Oral direct thrombin inhibitor AZD0837 for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: a randomized dose-guiding, safety, and tolerability study of four doses of AZD0837 vs. vitamin K antagonists
Abstract
Aims: Oral anticoagulation with vitamin K antagonists (VKAs) for stroke prevention in atrial fibrillation (AF) is effective but has significant limitations. AZD0837, a new oral anticoagulant, is a prodrug converted to a selective and reversible direct thrombin inhibitor (AR-H067637). We report from a Phase II randomized, dose-guiding study (NCT00684307) to assess safety, tolerability, pharmacokinetics, and pharmacodynamics of extended-release AZD0837 in patients with AF.
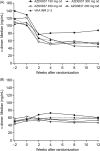
Methods and results: Atrial fibrillation patients (n = 955) with > or =1 additional risk factor for stroke were randomized to receive AZD0837 (150, 300, or 450 mg once daily or 200 mg twice daily) or VKA (international normalized ratio 2-3, target 2.5) for 3-9 months. Approximately 30% of patients were naïve to VKA treatment. Total bleeding events were similar or lower in all AZD0837 groups (5.3-14.7%, mean exposure 138-145 days) vs. VKA (14.5%, mean exposure 161 days), with fewer clinically relevant bleeding events on AZD0837 150 and 300 mg once daily. Adverse events were similar between treatment groups; with AZD0837, the most common were gastrointestinal disorders (e.g. diarrhoea, flatulence, or nausea). d-Dimer, used as a biomarker of thrombogenesis, decreased in all groups in VKA-naïve subjects with treatment, whereas in VKA pre-treated patients, d-dimer levels started low and remained low in all groups. As expected, only a few strokes or systemic embolic events occurred. In the AZD0837 groups, mean S-creatinine increased by approximately 10% from baseline and returned to baseline following treatment cessation. The frequency of serum alanine aminotransferase > or =3x upper limit of normal was similar for AZD0837 and VKA.
Conclusion: AZD0837 was generally well tolerated at all doses tested. AZD0837 treatment at an exposure corresponding to the 300 mg od dose in this study provides similar suppression of thrombogenesis at a potentially lower bleeding risk compared with dose-adjusted VKA. This study is registered with ClinicalTrials.gov, number NCT00684307.
Figures


Comment in
-
Direct thrombin inhibitors in atrial fibrillation reloaded.Eur Heart J. 2009 Dec;30(23):2832-4. doi: 10.1093/eurheartj/ehp320. Epub 2009 Aug 18. Eur Heart J. 2009. PMID: 19690350 No abstract available.
References
-
- Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6:981–993. - PubMed
-
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S Task Force on Practice Guidelines, American College of Cardiology/American Heart Association; Committee for Practice Guidelines, European Society of Cardiology; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation) Eur Heart J. 2006;27:1979–2030. - PubMed
-
- National Collaborating Centre for Chronic Conditions. Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians; 2006. - PubMed
-
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl. 3):204S–233S. - PubMed
-
- Choudhury A, Goyal D, Lip GY. Ximelagatran. Drugs Today (Barc) 2006;42:3–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

