Agarolytic bacterium Persicobacter sp. CCB-QB2 exhibited a diauxic growth involving galactose utilization pathway
- PMID: 27987272
- PMCID: PMC5300873
- DOI: 10.1002/mbo3.405
Agarolytic bacterium Persicobacter sp. CCB-QB2 exhibited a diauxic growth involving galactose utilization pathway
Abstract
The agarolytic bacterium Persicobacter sp. CCB-QB2 was isolated from seaweed (genus Ulva) collected from a coastal area of Malaysia. Here, we report a high-quality draft genome sequence for QB2. The Rapid Annotation using Subsystem Technology (RAST) annotation server identified four β-agarases (PdAgaA, PdAgaB, PdAgaC, and PdAgaD) as well as galK, galE, and phosphoglucomutase, which are related to the Leloir pathway. Interestingly, QB2 exhibited a diauxic growth in the presence of two kinds of nutrients, such as tryptone and agar. In cells grown with agar, the profiles of agarase activity and growth rate were very similar. galK, galE, and phosphoglucomutase genes were highly expressed in the second growth phase of diauxic growth, indicating that QB2 cells use galactose hydrolyzed from agar by its agarases and exhibit nutrient prioritization. This is the first report describing diauxic growth for agarolytic bacteria. QB2 is a potential novel model organism for studying diauxic growth in environmental bacteria.
Keywords: Persicobacter; agarase; agarolytic bacterium; diauxic growth; leloir pathway.
© 2016 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures

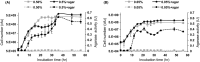


References
-
- Allouch, J. , Jam, M. , Helbert, W. , Barbeyron, T. , Kloareg, B. , Henrissat, B. , & Czjzek, M. (2003). The three‐dimensional structures of two β‐agarases. Journal of Biological Chemistry, 278, 47171–47180. - PubMed
-
- Aoki, T. , Araki, T. , & Kitamikado, M. (1990). Purification and characterization of a novel β‐agarase from Vibrio sp. AP‐2. European Journal of Biochemistry, 187, 461–465. - PubMed
-
- Araki, C. (1956). Structure of the agarose constituent of agar‐agar. Bulletin of the Chemical Society of Japan, 29, 543–544.
-
- Ariga, O. , Inoue, T. , Kubo, H. , Minami, K. , Nakamura, M. , Iwai, M. , … Nakasaki, K. (2012). Cloning of agarase gene from non‐marine agarolytic bacterium Cellvibrio sp. Journal of Microbiology and Biotechnology, 22, 1237–1244. - PubMed
-
- Aziz, R. K. , Bartels, D. , Best, A. A. , Dejongh, M. , Disz, T. , Edwards, R. A. , … Kubal, M. (2008). The RAST Server: Rapid annotations using subsystems technology. BMC Genomics, 9, 75. doi:10.1186/1471‐2164‐9‐75 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

