Evolution and Spread of Ebola Virus in Liberia, 2014-2015
- PMID: 26651942
- PMCID: PMC4711363
- DOI: 10.1016/j.chom.2015.11.008
Evolution and Spread of Ebola Virus in Liberia, 2014-2015
Abstract
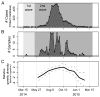
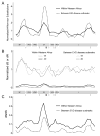
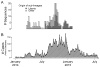
The 2013-present Western African Ebola virus disease (EVD) outbreak is the largest ever recorded with >28,000 reported cases. Ebola virus (EBOV) genome sequencing has played an important role throughout this outbreak; however, relatively few sequences have been determined from patients in Liberia, the second worst-affected country. Here, we report 140 EBOV genome sequences from the second wave of the Liberian outbreak and analyze them in combination with 782 previously published sequences from throughout the Western African outbreak. While multiple early introductions of EBOV to Liberia are evident, the majority of Liberian EVD cases are consistent with a single introduction, followed by spread and diversification within the country. Movement of the virus within Liberia was widespread, and reintroductions from Liberia served as an important source for the continuation of the already ongoing EVD outbreak in Guinea. Overall, little evidence was found for incremental adaptation of EBOV to the human host.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Albariño CG, Guerrero LW, Lo MK, Nichol ST, Towner JS. Development of a reverse genetics system to generate a recombinant Ebola virus Makona expressing a green fluorescent protein. Virology. 2015;484:259–264. - PubMed
-
- Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keita S, De Clerck H, et al. Emergence of Zaire Ebola virus disease in Guinea. The New England journal of medicine. 2014;371:1418–1425. - PubMed
-
- Bell A, Lewandowski K, Myers R, Wooldridge D, Aarons E, Simpson A, Vipond R, Jacobs M, Gharbia S, Zambon M. Genome sequence analysis of Ebola virus in clinical samples from three British healthcare workers, August 2014 to March 2015. 2015 ( http://www.eurosurveillance.org/images/dynamic/EE/V20N20/art21131.pdf) - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

