Clinical Impact of a Digital Dose Counter Pressurized Metered-Dose Inhaler on Uncontrolled Asthma: Cross-Sectional, Observational, Surveillance Study
- PMID: 31066694
- PMCID: PMC6528432
- DOI: 10.2196/13530
Clinical Impact of a Digital Dose Counter Pressurized Metered-Dose Inhaler on Uncontrolled Asthma: Cross-Sectional, Observational, Surveillance Study
Abstract
Background: In India, control of asthma with persistent symptoms remains a clinical enigma with likely incriminating factors including non- and pseudoadherence to the inhaled corticosteroids and long-acting beta2-agonists. The United States Food and Drug Administration guidance recommends the use of dose counter pressurized metered-dose inhalers (pMDIs) with further mechanisms to track adherence and pseudoadherence in real-world settings.
Objective: Digital dose counter pMDIs (dpMDIs) offer simplified, reliable tracking of individual "actuated" dosages with "END" display at completion of the labelled therapeutic aerosol spray. The translational impact on symptom persistence with likely unwarranted exposure to the "Step up" strategy is often prevented if not treated, as in the cases of "pseudo" severe asthma. To further assess the real-world acceptance and clinical impact of dpMDIs in bronchial asthma including poorly controlled or uncontrolled bronchial asthma cases, a noninterventional observational study was performed.
Methods: This cross-sectional, retrospective, case cohort, observational study-the Drug Utilization Surveillance-of dpMDIs in bronchial asthma was conducted in September 2016 in an outpatient setting in India. The retrospective analysis was initiated and conducted as per the International Conference on Harmonization Good Clinical Practice principles and Declaration of Helsinki, following approval from the local ethics committee and registration in the Clinical Trial Registry of India.
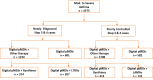
Results: Consecutive cases of moderate-to-severe asthma with poor control (n=4575), diagnosed as per the Global Initiative for Asthma symptom scale at baseline and follow-up, were included. Patients under treatment using dpMDIs were enrolled from 500 centers across India and assessed by respiratory care specialists. Baseline asthma control was assessed as partly controlled (n=4575) or poorly controlled (n=2942). Per protocol analyses showed that asthma was well controlled with dpMDIs at 8 weeks in 92.7% of the cases (2727/2942, P<.001). Adverse events (n=106, 2%) of mild-to-moderate intensity were reported. Nebulization was required in two patients with episodic breathlessness who were discharged with no consequent sequelae. Post hoc analyses for patients with baseline poorly controlled asthma who "switched" exclusively to dpMDI monotherapy or a combination with xanthines or long-acting beta2-agonists showed a "well controlled" asthma status in 85.9% (500/582, P=.04), 95.4% (395/414, P=.048), and 80.3% (106/132, P=.28) of the cases, respectively. The patient acceptability criteria for an "empty" canister was well correlated with the clinical strategy to identify and avoid pseudoadherence in poorly controlled or difficult-to-treat asthma cases, especially in patients who "switched" exclusively to dpMDIs (n=582) and demonstrated responses of "Use till twenty dose display" (65/156, 41.6%), "Use till END display" (83/156, 53.2%), and "Use till LAST spray" (8/156, 5.1%).
Conclusions: dpMDIs offer simple, accurate, and reliable tracking of non- and pseudoadherence while highlighting incremental asthma-control rates in severe and pseudosevere asthma cases before risk assessment for further "add-on" therapy.
Trial registration: Clinical Trials Registry - India CTRI/2018/06/014595; http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php? trialid=24583.
Keywords: anti-asthmatic agents; asthma; drug utilization surveillance; pseudoadherence; xanthines.
©Randeep Guleria, Krishnaprasad Korukonda, DUSS Investigators. Originally published in the Interactive Journal of Medical Research (http://www.i-jmr.org/), 05.05.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Gold LS, Thompson P, Salvi S, Faruqi RA, Sullivan SD. Level of asthma control and health care utilization in Asia-Pacific countries. Respir Med. 2014 Feb;108(2):271–7. doi: 10.1016/j.rmed.2013.12.004. https://linkinghub.elsevier.com/retrieve/pii/S0954-6111(13)00490-3 S0954-6111(13)00490-3 - DOI - PubMed
-
- Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010 Jun;19(116):150–7. doi: 10.1183/09059180.00002110. http://err.ersjournals.com/cgi/pmidlookup?view=long&pmid=20956184 19/116/150 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous