Effects of Selonsertib in Patients with Diabetic Kidney Disease
- PMID: 31506292
- PMCID: PMC6779369
- DOI: 10.1681/ASN.2018121231
Effects of Selonsertib in Patients with Diabetic Kidney Disease
Abstract
Background: Apoptosis signal-regulating kinase 1 (ASK1) activation in glomerular and tubular cells resulting from oxidative stress may drive kidney disease progression. Findings in animal models identified selonsertib, a selective ASK1 inhibitor, as a potential therapeutic agent.
Methods: In a phase 2 trial evaluating selonsertib's safety and efficacy in adults with type 2 diabetes and treatment-refractory moderate-to-advanced diabetic kidney disease, we randomly assigned 333 adults in a 1:1:1:1 allocation to selonsertib (oral daily doses of 2, 6, or 18 mg) or placebo. Primary outcome was change from baseline eGFR at 48 weeks.
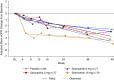
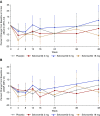
Results: Selonsertib appeared safe, with no dose-dependent adverse effects over 48 weeks. Although mean eGFR for selonsertib and placebo groups did not differ significantly at 48 weeks, acute effects related to inhibition of creatinine secretion by selonsertib confounded eGFR differences at 48 weeks. Because of this unanticipated effect, we used piecewise linear regression, finding two dose-dependent effects: an acute and more pronounced eGFR decline from 0 to 4 weeks (creatinine secretion effect) and an attenuated eGFR decline between 4 and 48 weeks (therapeutic effect) with higher doses of selonsertib. A post hoc analysis (excluding data for 20 patients from two sites with Good Clinical Practice compliance-related issues) found that between 4 and 48 weeks, rate of eGFR decline was reduced 71% for the 18-mg group relative to placebo (difference 3.11±1.53 ml/min per 1.73 m2 annualized over 1 year; 95% confidence interval, 0.10-6.13; nominal P=0.043). Effects on urine albumin-to-creatinine ratio did not differ between selonsertib and placebo.
Conclusions: Although the trial did not meet its primary endpoint, exploratory post hoc analyses suggest that selonsertib may slow diabetic kidney disease progression.
Keywords: chronic kidney disease; clinical trial; diabetes mellitus; glomerular filtration rate; oxidative stress.
Copyright © 2019 by the American Society of Nephrology.
Figures



References
-
- Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 - PubMed
-
- Umanath K, Lewis JB: Update on diabetic nephropathy: Core curriculum 2018. Am J Kidney Dis 71: 884–895, 2018 - PubMed
-
- Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J: Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol 10: 325–346, 2014 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

