A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells
- PMID: 9560222
- PMCID: PMC20207
- DOI: 10.1073/pnas.95.9.5027
A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells
Abstract
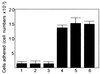
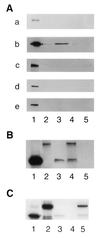
Trophinin and tastin form a cell adhesion molecule complex that potentially mediates an initial attachment of the blastocyst to uterine epithelial cells at the time of implantation. Trophinin and tastin, however, do not directly bind to each other, suggesting the presence of an intermediary protein. The present study identifies a cytoplasmic protein, named bystin, that directly binds trophinin and tastin. Bystin consists of 306 amino acid residues and is predicted to contain tyrosine, serine, and threonine residues in contexts conforming to motifs for phosphorylation by protein kinases. Database searches revealed a 53% identity of the predicted peptide sequence with the Drosophila bys (mrr) gene. Direct protein-protein interactions of trophinin, tastin, and bystin analyzed by yeast two-hybrid assays and by in vitro protein binding assays indicated that binding between bystin and trophinin and between bystin and tastin is enhanced when cytokeratin 8 and 18 are present as the third molecule. Immunocytochemistry of bystin showed that bystin colocalizes with trophinin, tastin, and cytokeratins in a human trophoblastic teratocarcinoma cell, HT-H. It is therefore possible that these molecules form a complex and thus are involved in the process of embryo implantation.
Figures


References
-
- Schlafke S, Enders A C. Biol Reprod. 1975;12:41–65. - PubMed
-
- Denker H W. In: Trophoblast Research. Denker H W, Aplin J D, editors. New York: Plenum; 1990. pp. 3–20.
-
- Psychoyos A. In: Handbook of Physiology. Greep R O, Astwood E G, Geige R G, editors. Washington, DC: Am. Physiol. Soc.; 1973. pp. 187–215.
-
- Day S K. In: Reproductive Endocrinology, Surgery and Technology. Adashi E Y, Rock J A, Rosenwaks Z, editors. Philadelphia: Lippincott–Raven; 1996. pp. 421–434.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

