The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer's disease
- PMID: 24252572
- PMCID: PMC3893579
- DOI: 10.1186/2051-5960-1-31
The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer's disease
Abstract
Background: Progressive supranuclear palsy (PSP) is a neurodegenerative disorder pathologically characterized by intracellular tangles of hyperphosphorylated tau protein distributed throughout the neocortex, basal ganglia, and brainstem. A genome-wide association study identified EIF2AK3 as a risk factor for PSP. EIF2AK3 encodes PERK, part of the endoplasmic reticulum's (ER) unfolded protein response (UPR). PERK is an ER membrane protein that senses unfolded protein accumulation within the ER lumen. Recently, several groups noted UPR activation in Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis, multiple system atrophy, and in the hippocampus and substantia nigra of PSP subjects. Here, we evaluate UPR PERK activation in the pons, medulla, midbrain, hippocampus, frontal cortex and cerebellum in subjects with PSP, AD, and in normal controls.
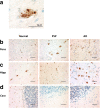
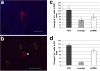
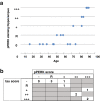
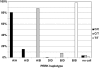
Results: We found UPR activation primarily in disease-affected brain regions in both disorders. In PSP, the UPR was primarily activated in the pons and medulla and to a much lesser extent in the hippocampus. In AD, the UPR was extensively activated in the hippocampus. We also observed UPR activation in the hippocampus of some elderly normal controls, severity of which positively correlated with both age and tau pathology but not with Aβ plaque burden. Finally, we evaluated EIF2AK3 coding variants that influence PERK activation. We show that a haplotype associated with increased PERK activation is genetically associated with increased PSP risk.
Conclusions: The UPR is activated in disease affected regions in PSP and the genetic evidence shows that this activation increases risk for PSP and is not a protective response.
Figures



References
-
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH. et al.Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;1:1–9. doi: 10.1212/WNL.47.1.1. - DOI - PubMed
-
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;1:333–359. doi: 10.1001/archneur.1964.00460160003001. - DOI - PubMed
-
- Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;1:663–672. - PubMed
-
- Stanford PM, Halliday GM, Brooks WS, Kwok JB, Storey CE, Creasey H, Morris JG, Fulham MJ, Schofield PR. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain. 2000;1:t 5:880–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0501560/MRC_/Medical Research Council/United Kingdom
- 1F31AG043254/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- F31 AG043254/AG/NIA NIH HHS/United States
- R37 AG011762/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- R37 AG11762/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- AG017586/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- P30 AG19610/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

