Lithium Treatment in the Prevention of Repeat Suicide-Related Outcomes in Veterans With Major Depression or Bipolar Disorder: A Randomized Clinical Trial
- PMID: 34787653
- PMCID: PMC8600458
- DOI: 10.1001/jamapsychiatry.2021.3170
Lithium Treatment in the Prevention of Repeat Suicide-Related Outcomes in Veterans With Major Depression or Bipolar Disorder: A Randomized Clinical Trial
Abstract
Importance: Suicide and suicide attempts are persistent and increasing public health problems. Observational studies and meta-analyses of randomized clinical trials have suggested that lithium may prevent suicide in patients with bipolar disorder or depression.
Objective: To assess whether lithium augmentation of usual care reduces the rate of repeated episodes of suicide-related events (repeated suicide attempts, interrupted attempts, hospitalizations to prevent suicide, and deaths from suicide) in participants with bipolar disorder or depression who have survived a recent event.
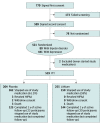
Design, setting, and participants: This double-blind, placebo-controlled randomized clinical trial assessed lithium vs placebo augmentation of usual care in veterans with bipolar disorder or depression who had survived a recent suicide-related event. Veterans at 29 VA medical centers who had an episode of suicidal behavior or an inpatient admission to prevent suicide within 6 months were screened between July 1, 2015, and March 31, 2019.
Interventions: Participants were randomized to receive extended-release lithium carbonate beginning at 600 mg/d or placebo.
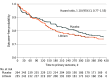
Main outcomes and measures: Time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
Results: The trial was stopped for futility after 519 veterans (mean [SD] age, 42.8 [12.4] years; 437 [84.2%] male) were randomized: 255 to lithium and 264 to placebo. Mean lithium concentrations at 3 months were 0.54 mEq/L for patients with bipolar disorder and 0.46 mEq/L for patients with major depressive disorder. No overall difference in repeated suicide-related events between treatments was found (hazard ratio, 1.10; 95% CI, 0.77-1.55). No unanticipated safety concerns were observed. A total of 127 participants (24.5%) had suicide-related outcomes: 65 in the lithium group and 62 in the placebo group. One death occurred in the lithium group and 3 in the placebo group.
Conclusions and relevance: In this randomized clinical trial, the addition of lithium to usual Veterans Affairs mental health care did not reduce the incidence of suicide-related events in veterans with major depression or bipolar disorders who experienced a recent suicide event. Therefore, simply adding lithium to existing medication regimens is unlikely to be effective for preventing a broad range of suicide-related events in patients who are actively being treated for mood disorders and substantial comorbidities.
Trial registration: ClinicalTrials.gov Identifier: NCT01928446.
Conflict of interest statement
Figures
Comment in
-
Testing for Antisuicidal Effects of Lithium Treatment.JAMA Psychiatry. 2022 Jan 1;79(1):9-10. doi: 10.1001/jamapsychiatry.2021.2992. JAMA Psychiatry. 2022. PMID: 34787652 No abstract available.
-
Suicide Risk and Lithium-Reply.JAMA Psychiatry. 2022 May 1;79(5):513-514. doi: 10.1001/jamapsychiatry.2022.0084. JAMA Psychiatry. 2022. PMID: 35262638 No abstract available.
-
Suicide Risk and Lithium.JAMA Psychiatry. 2022 May 1;79(5):513. doi: 10.1001/jamapsychiatry.2022.0081. JAMA Psychiatry. 2022. PMID: 35262644 No abstract available.
References
-
- Hedegaard H, Curtin SC, Warner M. Suicide Mortality in the United States, 1999–2017. National Center for Health Statistics Data Brief 330. National Center for Health Statistics; 2018.