Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial
- PMID: 23032552
- PMCID: PMC3564227
- DOI: 10.1001/jama.2012.12607
Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial
Abstract
Context: Nondystrophic myotonias (NDMs) are rare diseases caused by mutations in skeletal muscle ion channels. Patients experience delayed muscle relaxation causing functionally limiting stiffness and pain. Mexiletine-induced sodium channel blockade reduced myotonia in small studies; however, as is common in rare diseases, larger studies of safety and efficacy have not previously been considered feasible.
Objective: To determine the effects of mexiletine for symptoms and signs of myotonia in patients with NDMs.
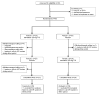
Design, setting, and participants: A randomized, double-blind, placebo-controlled 2-period crossover study at 7 neuromuscular referral centers in 4 countries of 59 patients with NDMs conducted between December 23, 2008, and March 30, 2011, as part of the National Institutes of Health-funded Rare Disease Clinical Research Network.
Intervention: Oral 200-mg mexiletine or placebo capsules 3 times daily for 4 weeks, followed by the opposite intervention for 4 weeks, with 1-week washout in between.
Main outcome measures: Patient-reported severity score of stiffness recorded on an interactive voice response (IVR) diary (scale of 1 = minimal to 9 = worst ever experienced). Secondary end points included IVR-reported changes in pain, weakness, and tiredness; clinical myotonia assessment; quantitative measure of handgrip myotonia; and Individualized Neuromuscular Quality of Life summary quality of life score (INQOL-QOL, percentage of maximal detrimental impact).
Results: Mexiletine significantly improved patient-reported severity score stiffness on the IVR diary. Because of a statistically significant interaction between treatment and period for this outcome, primary end point is presented by period (period 1 means were 2.53 for mexiletine and 4.21 for placebo; difference, -1.68; 95% CI, -2.66 to -0.706; P < .001; period 2 means were 1.60 for mexiletine and 5.27 for placebo; difference, -3.68; 95% CI, -3.85 to -0.139; P = .04). Mexiletine improved the INQOL-QOL score (mexiletine, 14.0 vs placebo, 16.7; difference, -2.69; 95% CI, -4.07 to -1.30; P < .001) and decreased handgrip myotonia on clinical examination (mexiletine, 0.164 seconds vs placebo, 0.494 seconds; difference, -0.330; 95% CI, -0.633 to -0.142; P < .001). The most common adverse effect was gastrointestinal (9 mexiletine and 1 placebo). Two participants experienced transient cardiac effects that did not require stopping the study (1 in each group). One serious adverse event was determined to be not study related.
Conclusion: In this preliminary study of patients with NDMs, the use of mexiletine compared with placebo resulted in improved patient-reported stiffness over 4 weeks of treatment, despite some concern about the maintenance of blinding.
Trial registration: clinicaltrials.gov Identifier: NCT00832000.
Figures



Comment in
-
Mexiletine for treatment of myotonia: a trial triumph for rare disease networks.JAMA. 2012 Oct 3;308(13):1377-8. doi: 10.1001/jama.2012.12906. JAMA. 2012. PMID: 23032555 Free PMC article. No abstract available.
References
-
- Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. - PubMed
-
- Leyburn P, Walton JN. The treatment of myotonia: a controlled clinical trial. Brain. 1959 Mar;82(1):81–91. - PubMed
-
- Griggs RC, Davis RJ, Anderson DC, Dove JT. Cardiac conduction in myotonic dystrophy. Am J Med. 1975 Jul;59(1):37–42. - PubMed
-
- Streib EW. Paramyotonia congenita: successful treatment with tocainide. Clinical and electrophysiologic findings in seven patients. Muscle Nerve. 1987 Feb;10(2):155–162. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- T32 NS07338-20/NS/NINDS NIH HHS/United States
- UL1 RR033179/RR/NCRR NIH HHS/United States
- R01 FD 003454/FD/FDA HHS/United States
- R01 FD003454/FD/FDA HHS/United States
- U54 NS059065-05S1/NS/NINDS NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- UL1 RR 024160/RR/NCRR NIH HHS/United States
- UL1 RR 024982/RR/NCRR NIH HHS/United States
- G0601943/MRC_/Medical Research Council/United Kingdom
- U54 NS059065/NS/NINDS NIH HHS/United States
- T32 NS007338/NS/NINDS NIH HHS/United States
- UL1 TR 000001/TR/NCATS NIH HHS/United States
- UL1 RR 033179/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

