Potassium Supplementation and Prevention of Atrial Fibrillation After Cardiac Surgery: The TIGHT K Randomized Clinical Trial
- PMID: 39215972
- PMCID: PMC11366075
- DOI: 10.1001/jama.2024.17888
Potassium Supplementation and Prevention of Atrial Fibrillation After Cardiac Surgery: The TIGHT K Randomized Clinical Trial
Abstract
Importance: Supplementing potassium in an effort to maintain high-normal serum concentrations is a widespread strategy used to prevent atrial fibrillation after cardiac surgery (AFACS), but is not evidence-based, carries risks, and is costly.
Objective: To determine whether a lower serum potassium concentration trigger for supplementation is noninferior to a high-normal trigger.
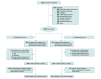
Design, setting, and participants: This open-label, noninferiority, randomized clinical trial was conducted at 23 cardiac surgical centers in the United Kingdom and Germany. Between October 20, 2020, and November 16, 2023, patients with no history of atrial dysrhythmias scheduled for isolated coronary artery bypass grafting (CABG) surgery were enrolled. The last study patient was discharged from the hospital on December 11, 2023.
Interventions: Patients were randomly assigned to a strategy of tight or relaxed potassium control (only supplementing if serum potassium concentration fell below 4.5 mEq/L or 3.6 mEq/L, respectively). Patients wore an ambulatory heart rhythm monitor, which was analyzed by a core laboratory masked to treatment assignment.
Main outcomes and measures: The prespecified primary end point was clinically detected and electrocardiographically confirmed new-onset AFACS in the first 120 hours after CABG surgery or until hospital discharge, whichever occurred first. All primary outcome events were validated by an event validation committee, which was masked to treatment assignment. Noninferiority of relaxed potassium control was defined as a risk difference for new-onset AFACS with associated upper bound of a 1-sided 97.5% CI of less than 10%. Secondary outcomes included other heart rhythm-related events, clinical outcomes, and cost related to the intervention.
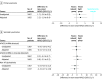
Results: A total of 1690 patients (mean age, 65 years; 256 [15%] females) were randomized. The primary end point occurred in 26.2% of patients (n = 219) in the tight group and 27.8% of patients (n = 231) in the relaxed group, which is a risk difference of 1.7% (95% CI, -2.6% to 5.9%). There was no difference between the groups in the incidence of at least 1 AFACS episode detected by any means or by ambulatory heart rhythm monitor alone, non-AFACS dysrhythmias, in-patient mortality, or length of stay. Per-patient cost for purchasing and administering potassium was significantly lower in the relaxed group (mean difference, $111.89 [95% CI, $103.60-$120.19]; P <.001).
Conclusions and relevance: For AFACS prophylaxis, supplementation only when serum potassium concentration fell below 3.6 mEq/L was noninferior to the current widespread practice of supplementing potassium to maintain a serum potassium concentration greater than or equal to 4.5 mEq/L. The lower threshold of supplementation was not associated with any increase in dysrhythmias or adverse clinical outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT04053816.
Conflict of interest statement
Figures

References
-
- Vervoort D, Lee G, Ghandour H, et al. . Global cardiac surgical volume and gaps: trends, targets, and way forward. Ann Thorac Surg Short Rep. 2024;2(2):320-324. doi:10.1016/j.atssr.2023.11.019 - DOI
-
- Mathew JP, Fontes ML, Tudor IC, et al. ; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group . A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720-1729. doi:10.1001/jama.291.14.1720 - DOI - PubMed
-
- O’Brien B, Burrage PS, Ngai JY, et al. . Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(1):12-26. doi:10.1053/j.jvca.2018.09.039 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

