Does participation in an HIV vaccine efficacy trial affect risk behaviour in South Africa?
- PMID: 23370155
- PMCID: PMC3942784
- DOI: 10.1016/j.vaccine.2013.01.031
Does participation in an HIV vaccine efficacy trial affect risk behaviour in South Africa?
Abstract
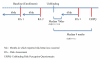
Background: Increased sexual risk behaviour in participants enrolled in HIV prevention trials has been a concern. The HVTN 503/Phambili study, a phase 2B study of the Merck Ad-5 multiclade HIV vaccine in South Africa, suspended enrollment and vaccinations following the results of the Step study. Participants were notified of their treatment allocation and continue to be followed. We investigated changes in risk behaviour over time and assessed the impact of study unblinding.
Methods: 801 participants were enrolled. Risk behaviours were assessed with an interviewer-administered questionnaire at 6-month intervals. We assessed change from enrolment to the first 6-month assessment pre-unblinding and between enrolment and at least 6 months post-unblinding on all participants with comparable data. A one-time unblinding risk perception questionnaire was administered post-unblinding.
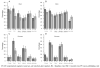
Results: A decrease in participants reporting unprotected sex was observed in both measured time periods for men and women, with no differences by treatment arm. At 6 months (pre-unblinding), 29.6% of men and 35.8% of women reported changing from unprotected to protected sex (p<0.0001 for each). Men (22%) were more likely than women (14%) to report behaviour change after unblinding (p=0.009). Post-enrolment, 142 (45%) of 313 previously uncircumcised men underwent medical circumcision. 663 participants completed the unblinding questionnaire. More vaccine (24.6%) as compared to placebo recipients (12.0%) agreed that they were more likely to get HIV than most people (p<0.0001), and attributed this to receiving the vaccine.
Conclusion: We did not find evidence of risk compensation during this clinical trial. Some risk behaviour reductions including male circumcision were noted irrespective of treatment allocation.
Copyright © 2013. Published by Elsevier Ltd.
Figures
References
-
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. on behalf of The HVTN 503/Phambili study team Safety and efficacy of the HVTN 503/Phambili Study of a clade-B-based HIV-1 vaccine in South Africa: a double blind, Randomized, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011 Jul;11(7):507–515. Epub 2011 May 11. PubMed PMID: 21570355. - PMC - PubMed
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy Assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–93. Epub 2008 Nov 13. PubMed PMID: 19012954; PubMed Central PMCID: PMC2721012. - PMC - PubMed
-
- Simon AE, Wu AW, Lavori PW, Sugarman J. Preventive misconception. Its nature, presence and ethical implications for research. Am J Prev Med. 2007;32(5):370–374. - PubMed
-
- Pinkerton SD. Sexual risk compensation and HIV/STD transmission: empirical evidence and theoretical considerations. Risk Anal. 2001 Aug;21(4):727–36. PubMed PMID: 11726023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources