Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study
- PMID: 20835873
- PMCID: PMC3166600
- DOI: 10.1007/s00520-010-0990-y
Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study
Abstract
Purpose: A novel transdermal formulation of granisetron (the granisetron transdermal delivery system (GTDS)) has been developed to deliver granisetron continuously over 7 days. This double-blind, phase III, non-inferiority study compared the efficacy and tolerability of the GTDS to daily oral granisetron for the control of chemotherapy-induced nausea and vomiting (CINV).
Patients and methods: Six hundred forty-one patients were randomized to oral (2 mg/day, 3-5 days) or transdermal granisetron (one GTDS patch, 7 days), before receiving multi-day chemotherapy. The primary endpoint was complete control of CINV (no vomiting/retching, no more than mild nausea, no rescue medication) from chemotherapy initiation until 24 h after final administration. The prespecified non-inferiority margin was 15%.
Results: Five hundred eighty-two patients were included in the per protocol analysis. The GTDS displayed non-inferiority to oral granisetron: complete control was achieved by 60% of patients in the GTDS group, and 65% in the oral granisetron group (treatment difference, -5%; 95% confidence interval, -13-3). Both treatments were well tolerated, the most common adverse event being constipation.
Conclusions: The GTDS provides effective, well-tolerated control of CINV associated with moderately or highly emetogenic multi-day chemotherapy. It offers a convenient alternative route for delivering granisetron for up to 7 days that is as effective as oral granisetron.
Trial registration: ClinicalTrials.gov NCT00273468.
Figures
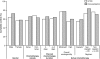
References
-
- Hainsworth JD. The use of ondansetron in patients receiving multiple-day cisplatin regimens. Semin Oncol. 1992;19:48–52. - PubMed
-
- Dearnaley DP, Judson I, Root T (2002) Handbook of adult cancer chemotherapy schedules, 2nd edn. Oxon, UK, TMG Healthcare Communications, in collaboration with The Royal Marsden NHS Trust
-
- Herrstedt J, Sigsgaard TC, Nielsen HA, Handberg J, Langer SW, Ottesen S, Dombernowsky P. Randomized, double-blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple-day cisplatin-based chemotherapy. Support Care Cancer. 2007;15:417–426. doi: 10.1007/s00520-006-0158-y. - DOI - PubMed
-
- Navari RM. Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J Natl Compr Canc Netw. 2007;5:51–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

