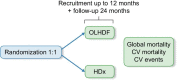
Trial design of the MOTheR HDx study: a multicenter, open-label, prospective, randomized study to explore the morbidity and mortality in patients dialyzed with the Theranova HDx in comparison with online hemodiafiltration
- PMID: 37915938
- PMCID: PMC10616438
- DOI: 10.1093/ckj/sfad128
Trial design of the MOTheR HDx study: a multicenter, open-label, prospective, randomized study to explore the morbidity and mortality in patients dialyzed with the Theranova HDx in comparison with online hemodiafiltration
Abstract
Background: Dialysis patients have been maintaining a high rate of cardiovascular morbidity and mortality. For this reason, it is to introduce necessary new technical advances in clinical practice. There is a relation between toxins retention and inflammation, mortality and morbidity. Medium cut-off (MCO) membranes are a new generation of membranes that allow the removal of a greater number of medium-sized molecules compared with high-flux hemodialysis (HF-HD), but retaining albumin. MCO membranes have an increased permeability and the presence of internal filtration. Because of these special properties, MCO generated a new concept of therapy called expanded HD (HDx). Until now, online hemodiafiltration (OL-HDF) has demonstrated its superiority, in terms of survival, compared with HF-HD. However, the comparison between OL-HDF and HDx remains an unsolved question.
Methods: The MOTheR HDx study trial (NCT03714386) is an open-label, multicenter, prospective, 1:1 randomized, parallel-group trial designed to evaluate the efficacy and safety of HDx compared with OL-HDF in patients treated for dialysis in Spain for up to 36 months. The main endpoint is to determinate whether HDx is non inferior to OL-HDF at reducing the combined outcome of all-cause death and stroke (ischemic or hemorrhagic), acute coronary syndrome (angina and myocardial infarction), peripheral arterial disease (amputation or revascularization) and ischemic colitis (mesenteric thrombosis).
Results: The trial has already started.
Keywords: cardiovascular events; expanded hemodialysis (HDx); medium cut-off membranes (MCO); mortality; online hemodiafiltration (OL-HDF).
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
P.d.S. reports honorarium for conferences, consulting fees and advisory boards from Amgen, Astellas, AstraZeneca, Baxter, Braun, Fresenius Medical Care, GlaxoSmithKline, Nipro, Otsuka, Sandoz, Nipro and Vifor-Pharma. She is the present president of the Spanish Society of Nephrology (S.E.N.). R.P.-G. reports honorarium from Nipro. A.V. has received consultancy fees and lecture fees from Baxter, Braun and Astellas. P.M.-M. report honorarium for conferences and consulting fee from Nipro. M.F.-L. reports honorarium for conferences from Nipro. E.C. reports honorarium for conferences from Fresenius, Astellas and AstraZeneca, and studies from Baxter. I.C. reports honorarium for conferences from Braun, Palex and Vifor Pharma. F.M. has received consultancy fees and lecture fees from Baxter, Fresenius Medical Care, Medtronic, Nipro, Toray and Vifor. K.P.d.V., A.L.G.-H., J.G., M.A.G.-R., E.M. and B.G.-C. have no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous