Impaired immunity to recall antigens and neoantigens in severely immunocompromised children and adolescents during the first year of effective highly active antiretroviral therapy
- PMID: 18752430
- PMCID: PMC3895909
- DOI: 10.1086/592050
Impaired immunity to recall antigens and neoantigens in severely immunocompromised children and adolescents during the first year of effective highly active antiretroviral therapy
Abstract
Background: We studied whether severely immunocompromised, human immunodeficiency virus (HIV)-infected children who were beginning highly active antiretroviral therapy (HAART) or changing HAART regimens could spontaneously respond to a recall antigen (tetanus toxoid [TT] vaccine) or respond to a recall antigen and neoantigen (hepatitis A virus [HAV] vaccine) after 3 vaccinations.
Methods: A total of 46 children who had CD4 cell percentages <15% and who demonstrated a >0.75-log reduction in plasma HIV RNA levels within 4 weeks of starting HAART were randomized to receive vaccinations with either TT or HAV vaccines during the first 6 months of HAART. Study subjects then received the alternate vaccine during the next 6 months of HAART.
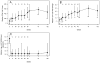
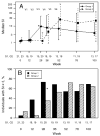
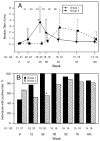
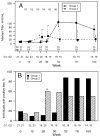
Results: Despite the early decline in viremia and the later increase in the percentage of CD4 T cells, spontaneous recovery of cell-mediated immunity (CMI) was not seen for TT. Serologic responses to TT required 3 vaccinations and were comparable in both groups. Serologic responses to HAV were infrequent and of low titer, although the group that received HAV vaccine after receiving TT vaccine performed somewhat better. CMI to HAV was virtually absent.
Conclusions: Severely immunocompromised children who are receiving HAART develop CMI and antibody to a recall antigen independent of the timing of vaccination, but they require a primary series of vaccinations. Antibodies to a neoantigen, HAV, developed when vaccination was delayed after initiation of HAART. CMI to a neoantigen was difficult to establish.
Trial registration: Clinicaltrials.gov identifier: NCT00004735/PACTG P1006 .
Conflict of interest statement
Potential conflicts of interest: none reported.
Figures
References
-
- Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–8. - PubMed
-
- Sanchez JM, Ramos Amador JT, Fernandez de Miguel S, et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:863–7. - PubMed
-
- Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, Seage GR., 3rd Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:330–1. - PubMed
-
- Viani RM, Arenata MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–31. - PubMed
-
- Resino S, Bellon JM, Resino R, et al. Extensive implementation of highly active antiretroviral therapy shows great effect on survival and surrogate markers in vertically HIV-infected children. Clin Infect Dis. 2004;38:1605–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

