Prospective study confirms oxandrolone-associated improvement in height in growth hormone-treated adolescent girls with Turner syndrome
- PMID: 20733274
- PMCID: PMC7903863
- DOI: 10.1159/000317529
Prospective study confirms oxandrolone-associated improvement in height in growth hormone-treated adolescent girls with Turner syndrome
Abstract
Background/aims: Untreated girls with Turner syndrome (TS) have growth failure, and adult height is, on average, 20 cm less than predicted height. Treatment with growth hormone (GH) is now standard of care. The objective of this study was to investigate the benefit of adding oxandrolone (Ox) to GH in a long-term, randomized, placebo (Pl)-controlled prospective trial to near adult height in TS.
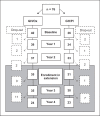
Methods: prospective, randomized, Pl-controlled study: 76 girls with TS (ages 10-14.9 years) were randomized to receive Ox (0.06 mg/kg/day) or Pl in combination with GH (0.35 mg/kg/week, daily) over 2 years. Auxologic data, breast and pubic hair Tanner stages, and hormone and lipid levels were measured. Subjects who chose to continue were followed in a 2-year double-blind extension, also received estrogen therapy (years 3, 4), and had dual-energy X-ray absorptiometry evaluation of bone density (years 3, 4).
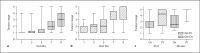
Results: at year 4, the change in absolute height and height SDS was greater in the GH/Ox versus GH/Pl group [26.2 ± 6.7 vs. 22.2 ± 5.1 cm, analysis of covariance (ANCOVA) p < 0.001; 1.8 ± 0.9 vs. 1.2 ± 0.7 standard deviation scores, ANCOVA p < 0.001]. Bone mineral density (BMD) of the wrist (0.51 ± 0.17 vs. 0.54 ± 0.05 g/cm(2)) and spine (0.91 ± 0.34 vs. 0.96 ± 0.13 g/cm(2)) in the GH/Ox versus GH/Pl groups was similar after 4 years. Breast development was slower in the GH/Ox versus GH/Pl group [year 4: Tanner stage 2.9 ± 1.3 (Ox) vs. 4.1 ± 1.3 (Pl), p = 0.003], and menarche was approximately 1 year later.
Conclusions: the addition of Ox to GH at mean age 12.0 ± 1.7 year augmented height gain after 4 years of treatment, slowed breast development and did not affect BMD in girls with TS. Whether initiation of Ox prior to initiation of pubertal development would optimize height gain without impeding breast development will require further study.
Trial registration: ClinicalTrials.gov NCT00029159.
2010 S. Karger AG, Basel.
Conflict of interest statement
M.Z., K.S., K.K., and H.K. have nothing to disclose. G.B.C is a former employee, retiree (with pension) and current consultant of Eli Lilly & Company. J.L.R. has received grant support from Eli Lilly & Company, Pfizer, and Novo Nordisk and has served as a consultant for Eli Lilly & Company, Pfizer, and Novo Nordisk.
Figures

Comment in
-
Comments on 'Prospective study confirms oxandrolone-associated improvement in height in growth hormone-treated adolescent girls with Turner syndrome' by Zeger et al., pp. 39-47, this issue.Horm Res Paediatr. 2011;75(1):47-8. doi: 10.1159/000317530. Epub 2010 Nov 3. Horm Res Paediatr. 2011. PMID: 21051854 No abstract available.
References
-
- Bareille P, Massarano AA, Stanhope R. Final height outcome in girls with Turner syndrome treated with a combination of low dose oestrogen and oxandrolone. Eur J Pediatr. 1997;156:358–362. - PubMed
-
- Crock P, Werther GA, Wettenhall HN. Oxandrolone increases final height in Turner syndrome. J Paediatr Child Health. 1990;26:221–224. - PubMed
-
- Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. - PubMed
-
- Urban MD, Lee PA, Dorst JP, Plotnick LP, Migeon CJ. Oxandrolone therapy in patients with Turner syndrome. J Pediatr. 1979;94:823–827. - PubMed

