Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer
- PMID: 34037241
- PMCID: PMC8150746
- DOI: 10.1002/14651858.CD011220.pub2
Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer
Abstract
Background: Retrospective analyses suggest that capecitabine may carry superior activity in hormone receptor-positive relative to hormone receptor-negative metastatic breast cancer. This review examined the veracity of that finding and explored whether this differential activity extends to early breast cancer.
Objectives: To assess effects of chemotherapy regimens containing capecitabine compared with regimens not containing capecitabine for women with hormone receptor-positive versus hormone receptor-negative breast cancer across the three major treatment scenarios: neoadjuvant, adjuvant, metastatic.
Search methods: On 4 June 2019, we searched the Cochrane Breast Cancer Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5) in the Cochrane Library; MEDLINE; Embase; the World Health Organization International Clinical Trials Registry Platform; and ClinicalTrials.gov.
Selection criteria: Randomised controlled trials looking at chemotherapy regimens containing capecitabine alone or in combination with other agents versus a control or similar regimen without capecitabine for treatment of breast cancer at any stage. The primary outcome measure for metastatic and adjuvant trials was overall survival (OS), and for neoadjuvant studies pathological complete response (pCR).
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias and certainty of evidence using the GRADE approach. Hazard ratios (HRs) were derived for time-to-event outcomes, and odds ratios (ORs) for dichotomous outcomes, and meta-analysis was performed using a fixed-effect model.
Main results: We included 26 studies with outcome data by hormone receptor: 12 metastatic studies (n = 4325), 6 neoadjuvant trials (n = 3152), and 8 adjuvant studies (n = 13,457). Capecitabine treatment was added in several different ways across studies. These could be classified as capecitabine alone compared to another treatment, capecitabine substituted for part of the control chemotherapy, and capecitabine added to control chemotherapy. In the metastatic setting, the effect of capecitabine was heterogenous between hormone receptor-positive and -negative tumours. For OS, no difference between capecitabine-containing and non-capecitabine-containing regimens was observed for all participants taken together (HR 1.01, 95% confidence interval (CI) 0.98 to 1.05; 12 studies, 4325 participants; high-certainty evidence), for those with hormone receptor-positive disease (HR 0.93, 95% CI 0.84 to 1.04; 7 studies, 1834 participants; high-certainty evidence), and for those with hormone receptor-negative disease (HR 1.00, 95% CI 0.88 to 1.13; 8 studies, 1577 participants; high-certainty evidence). For progression-free survival (PFS), a small improvement was seen for all people (HR 0.89, 95% CI 0.82 to 0.96; 12 studies, 4325 participants; moderate-certainty evidence). This was largely accounted for by a moderate improvement in PFS for inclusion of capecitabine in hormone receptor-positive cancers (HR 0.82, 95% CI 0.73 to 0.91; 7 studies, 1594 participants; moderate-certainty evidence) compared to no difference in PFS for hormone receptor-negative cancers (HR 0.96, 95% CI 0.83 to 1.10; 7 studies, 1122 participants; moderate-certainty evidence). Quality of life was assessed in five studies; in general there did not seem to be differences in global health scores between the two treatment groups at around two years' follow-up. Neoadjuvant studies were highly variable in design, having been undertaken to test various experimental regimens using pathological complete response (pCR) as a surrogate for disease-free survival (DFS) and OS. Across all patients, capecitabine-containing regimens resulted in little difference in pCR in comparison to non-capecitabine-containing regimens (odds ratio (OR) 1.12, 95% CI 0.94 to 1.33; 6 studies, 3152 participants; high-certainty evidence). By subtype, no difference in pCR was observed for either hormone receptor-positive (OR 1.22, 95% CI 0.76 to 1.95; 4 studies, 964 participants; moderate-certainty evidence) or hormone receptor-negative tumours (OR 1.28, 95% CI 0.61 to 2.66; 4 studies, 646 participants; moderate-certainty evidence). Four studies with 2460 people reported longer-term outcomes: these investigators detected no difference in either DFS (HR 1.02, 95% CI 0.86 to 1.21; high-certainty evidence) or OS (HR 0.97, 95% CI 0.77 to 1.23; high-certainty evidence). In the adjuvant setting, a modest improvement in OS was observed across all participants (HR 0.89, 95% CI 0.81 to 0.98; 8 studies, 13,547 participants; moderate-certainty evidence), and no difference in OS was seen in hormone receptor-positive cancers (HR 0.86, 95% CI 0.68 to 1.09; 3 studies, 3683 participants), whereas OS improved in hormone receptor-negative cancers (HR 0.72, 95% CI 0.59 to 0.89; 5 studies, 3432 participants). No difference in DFS or relapse-free survival (RFS) was observed across all participants (HR 0.93, 95% CI 0.86 to 1.01; 8 studies, 13,457 participants; moderate-certainty evidence). As was observed for OS, no difference in DFS/RFS was seen in hormone receptor-positive cancers (HR 1.03, 95% CI 0.91 to 1.17; 5 studies, 5604 participants; moderate-certainty evidence), and improvements in DFS/RFS with inclusion of capecitabine were observed for hormone receptor-negative cancers (HR 0.74, 95% CI 0.64 to 0.86; 7 studies, 3307 participants; moderate-certainty evidence). Adverse effects were reported across all three scenarios. When grade 3 or 4 febrile neutropenia was considered, no difference was seen for capecitabine compared to non-capecitabine regimens in neoadjuvant studies (OR 1.31, 95% CI 0.97 to 1.77; 4 studies, 2890 participants; moderate-certainty evidence), and a marked reduction was seen for capecitabine in adjuvant studies (OR 0.55, 95% CI 0.47 to 0.64; 5 studies, 8086 participants; moderate-certainty evidence). There was an increase in diarrhoea and hand-foot syndrome in neoadjuvant (diarrhoea: OR 1.95, 95% CI 1.32 to 2.89; 3 studies, 2686 participants; hand-foot syndrome: OR 6.77, 95% CI 4.89 to 9.38; 5 studies, 3021 participants; both moderate-certainty evidence) and adjuvant trials (diarrhoea: OR 2.46, 95% CI 2.01 to 3.01; hand-foot syndrome: OR 13.60, 95% CI 10.65 to 17.37; 8 studies, 11,207 participants; moderate-certainty evidence for both outcomes).
Authors' conclusions: In summary, a moderate PFS benefit by including capecitabine was seen only in hormone receptor-positive cancers in metastatic studies. No benefit of capecitabine for pCR was noted overall or in hormone receptor subgroups when included in neoadjuvant therapy. In contrast, the addition of capecitabine in the adjuvant setting led to improved outcomes for OS and DFS in hormone receptor-negative cancer. Future studies should stratify by hormone receptor and triple-negative breast cancer (TNBC) status to clarify the differential effects of capecitabine in these subgroups across all treatment scenarios, to optimally guide capecitabine inclusion.
Trial registration: ClinicalTrials.gov NCT00309556 NCT01642771 NCT00191438 NCT02748213 NCT00717951 NCT00114816 NCT00129935 NCT00196859 NCT00191152 NCT00301925 NCT00600340 NCT00089479 NCT01231802 NCT00024102 NCT00388726 NCT00049660 NCT00480597 NCT00209092 NCT00544765 NCT01050322 NCT00429871 NCT01204437 NCT00050167 NCT00082095 NCT01112826 NCT01354522 NCT01415336 NCT00266799 NCT01131195 NCT01433614 NCT01013740 NCT00196872.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SH: none related to this review. Received travel and accommodation funding for investigator meetings on unrelated trials (MK3475‐716, MK3475‐495, M16‐289) from Merck and AbbVie.
PL: none related to this review. Received financial support to attend educational events from Roche, Pfizer and Medical Oncology Group of Australia (MOGA), and honoraria for speaking at education meetings unrelated to this submitted work from Bristol‐Myers Squibb and Pfizer.
AW: none known.
MB: none known.
PB: none known.
AR: none related to this review. Received payment for developing education meeting on biosimilars from Roche, financial support for virtual registration at San Antonio Breast Cancer Symposium from Novartis and board membership from Roche, Novartis and Pfizer, and honoraria for unrelated research from Eisai.
Figures

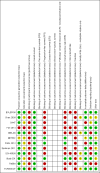
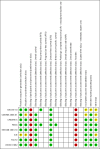
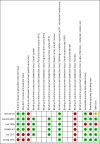
































































































Update of
References
References to studies included in this review
ABCSG‐24 {published data only}
-
- Foedermayr M, Sebesta M, Rudas M, Berghoff AS, Promberger R, Preusser M, et al. Abstract P1-08-40: BRCA-1 promotor methylation and p53 mutation in triple-negative breast cancer patients refractory to taxane-based neoadjuvant chemotherapy. Cancer Research 2013;73(24 Supplement):P1-08-40. [DOI: 10.1158/0008-5472.sabcs13-p1-08-40] - DOI - PubMed
-
- Steger G, Greil R, Jakesz R, Lang A, Mlineritsch B, Melbinger-Zeinitzer E, et al. Final results of ABCSG-24, a randomized phase III study comparing epirubicin, docetaxel, and capecitabine (EDC) to epirubicin and docetaxel (ED) as neoadjuvant treatment for early breast cancer and comparing ED/EDC + trastuzumab (T) to ED/EDC as neoadjuvant treatment for early HER-2 positive breast cancer. Cancer Research 2009;69(24 Suppl):Abstract no. 1081.
-
- Steger GG, Greil R, Jakesz R, Lang A, Mlineritsch B, Melbinger-Zeinitzer E et al. A randomized phase III study comparing epirubicin, docetaxel, and capecitabine (EDC) to epirubicin and docetaxel (ED) as neoadjuvant treatment for early breast cancer - First results of the Austrian breast and colorectal cancer study group-trial 24 (ABCSG-24). European Journal of Cancer Supplement 2009;7(2-3):3.
-
- Steger GG, Greil R, Jakesz R, Lang A, Mlineritsch B, Rudas M, et al. Independent prognostic factors for response: updated results of the ABCSG-24 study evaluating the addition of capecitabine to epirubicin-docetaxel neoadjuvant therapy for early breast cancer (EBC). European Journal of Cancer Supplement 2010;8(3):60.
-
- Steger GG, Greil R, Jakesz R, Lang A, Mlineritsch B, Rudas M, et al. Pathologic complete response (pCR) in patient subgroups: an analysis of ABCSG-24, a phase III, randomized study of anthracycline- and taxane-based neoadjuvant therapy with or without capecitabine in early breast cancer (EBC). In: Journal of Clinical Oncology. Vol. 28. 2010:Abstract no. 530.
BOLERO6 {published data only}
-
- Jerusalem G, Boer RH, Hurvitz S, Yardley DA, Kovalenko E, Ejlertsen B, et al. Everolimus plus exemestane vs everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-negative advanced breast cancer: the BOLERO-6 randomized clinical trial. JAMA Oncology 2018;4(10):1367-74. - PMC - PubMed
-
- Jerusalem GHM, Kovalenko E, Yardley DA, De Boer RH, Hurvitz SA, Ejlertsen B, et al. Everolimus (EVE) + exemestane (EXE) vs EVE alone or capecitabine (CAP) for estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): BOLERO-6, an open-label phase 2 study. In: Journal of Clinical Oncology. Vol. 36. 2018:Abstract no. 1005.
CBCSG‐10 {published data only}
-
- Li J, Shao Z, Yang S, Jiang J, Wang C, Liu Y, et al. CBCSG-10, the addition of capecitabine to adjuvant chemotherapy in triple-negative breast cancer. In: Breast. Vol. 24. 2015:S53. [DOI: ]
-
- Zhimin S, Li J, Pang D, Wang C, Jian J, Yang S, et al. CBCSG-10: adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for triple negative breast cancer. In: ASCO Meeting Abstracts. Vol. 34. 2016:1012.
Chan 2009 {published data only}
-
- Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Lluch A, et al. Gemcitabine plus docetaxel (GD) versus capecitabine plus docetaxel (CD) for anthracycline-pretreated metastatic breast cancer (MBC) patients (pts): results of a European phase III study. Journal of Clinical Oncology 2005;23(16 Suppl):24S. - PubMed
-
- Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, et al. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. Journal of Clinical Oncology 2009;27(11):1753-60. [DOI: 10.1200/JCO.2007.15.8485] - DOI - PubMed
-
- Fumoleau P, Romieu G, Chan S, Huober J, Tubiana-Julin M, Schneeweiss A, et al. Impact of symptoms and toxicity on quality of life: exploratory analysis of gemcitabine plus docetaxel vs capecitabine plus docetaxel in metastatic breast cancer. Journal of Clinical Oncology 2006;24(18 Suppl):48s.
-
- Levy C, Fumoleau P. Gemcitabine plus docetaxel: a new treatment option for anthracycline pretreated metastatic breast cancer patients? Cancer Treatment Reviews 2005;31(4 Suppl):S17-22. - PubMed
CHAT {published data only}
-
- Bell R, Anton-Torres A, Jassem J, Morales S, Cameron D, Button P, et al. Beyond CHAT: overall survival (OS) update from the chat study of first-line trastuzumab (H) plus docetaxel (T) with or without capecitabine (X) in HER2-positive metastatic breast cancer (MBC). Annals of Oncology 2010;21(Suppl 8):viii99.
-
- Wardlev A, Anton-Torres A, Pivot X, Morales-Vasquez F, Zetina L, Fatima Dias Gaui M, et al. Trastuzumab plus docetaxel with or without capecitabine as first-line therapy for HER2-positive locally advanced or metastatic breast cancer: a randomised Phase II study. EJC Supplements 2008;6(7):109-10. [DOI: 10.1016/S1359-6349(08)70525-1] - DOI
-
- Wardley A, Anton-Torres A, Otero Reyes D, Jassem J, Toache LMZ, Alcedo JC. CHAT - an open-label, randomised phase II study of trastuzumab plus deocetaxel with or without capecitabine in patients with advanced and/or metastatic, HER2-positive breast cancer: second interim safety analysis [abstract no: 1997] 1997. Breast Cancer Research & Treatment 2005;94(Suppl 1):S281-81.
-
- Wardley A, Anton-Torres A, Pivot X, Morales-Vasquez F, Zetina L, Fatima Dias Gaui M, et al. Evaluation of trastuzumab, docetaxel and capecitabine as first-line therapy for HER2-positive locally advanced or metastatic breast cancer. In: Breast Cancer Research and Treatment. Vol. Suppl. 2007:Abstract no. 309.
-
- Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fatima Dias, Gaui M, et al. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. Journal of Clinical Oncology 2010;28(6):976-83. - PubMed
CIBOMA 2004‐01 {published data only}
-
- Barrios CE, Lluch A, Ruiz-Borrego M, Bines J, Torrecillas L, Carrasco E, et al. Abstract OT3-1-06: CIBOMA/2004-01_GEICAM/2003-11: A randomised phase III trial assessing adjuvant capecitabine (Cap) maintenance therapy after standard chemotherapy for triple-negative early breast cancer. Cancer Research 2013;73(24 Suppl):OT3-1-06. [DOI: 10.1158/0008-5472.SABCS13-OT3-1-06] - DOI
-
- Lluch A, Gomez H, Ruiz-Borrego M, Bines J, Llombart A, Ramos M, et al. Abstract P5-10-15: First safety data from a randomised phase III (CIBOMA 2004- 01/GEICAM 2003-11) trial assessing adjuvant capecitabine maintenance therapy after standard chemotherapy for triple-negative early breast cancer. Cancer Research 2010;70(24 Suppl):P5-10-5-P5--5.
-
- Lluch A, Ruiz-Borrego M, Barrios CH, Bines J, Torrecillas L, Segalla JGM, et al. 422 Final safety data from a randomised phase III trial (CIBOMA/2004-01_GEICAM/2003-11) assessing adjuvant capecitabine maintenance therapy after standard chemotherapy for triple-negative early breast cancer; a study From Coalicion Iberoamericana De Investigacion En Oncologia Mamaria (CIBOMA) and Grupo Espanol De Investigacion En Cancer De Mama (GEICAM). European Journal of Cancer 2012;48(1):S169-70.
CREATE‐X {published data only}
-
- Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. New England Journal of Medicine 2017;376(22):2147-59. - PubMed
-
- Ohtani S, Masuda N, Im Y-H, Im S-A, Park B-W, Kim S-B, et al. Abstract P3-12-03: Adjuvant capecitabine in breast cancer patients with pathologic residual disease after neoadjuvant chemotherapy: first safety analysis of CREATE-X (JBCRG-04). Cancer Research 2013;73(24 Suppl):P3-12-03. [DOI: 10.1158/0008-5472.sabcs13-p3-12-03] - DOI
-
- Toi M, Lee SJ, Lee ES, Ohtani S, Im YH, Im SA, et al. A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive disease after neoadjuvant chemotherapy (CREATE-X, JBCRG-04). Cancer Research 2016;76(4 Suppl 1):Abstract S1-07. [DOI: ]
Fan 2013 {published data only}
-
- Fan Y, Xu BH, Yuan P, Wang JY, Ma F, Ding XY, et al. Results of a randomized phase II study demonstrate benefit of platinum-based regimen in the first-line treatment of triple negative breast cancer (TNBC). Cancer Research 2011;71(24):Abstract no. P5-19-04.
FINXX {published data only}01‐2003
-
- Joensuu H, Hemminki A, Tanner M, Paija O, Kokko R, Asolak R, et al. Phase III, randomized study of docetaxel (T) + capecitabine (X) (TX) followed by cyclophosphamide (C) + epirubicin (E) + X (CEX) vs. T followed by C + E + fluorouracil (F) (CEF) as adjuvant treatment for patients (pts) with early breast cancer (BC): an interim safety analysis. Journal of Clinical Oncology 2005;23(16 Suppl):57S.
-
- Joensuu H, Kellokumpu-Lehtinen P, Huovinen R, Jukkola-Vuorinen A, Asola R, Kokko R, et al. Significant improvement in recurrence-free survival (RFS) when capecitabine (X) is integrated into docetaxel (T) -> 5-FU + epirubicin + cyclophosphamide (CEF) adjuvant therapy for high-risk early breast cancer (BC): interim analysis of the FinXX-trial. Cancer Research 2009;69(2 Suppl):Abstract no. 82.
-
- Joensuu H, Kellokumpu-Lehtinen P, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, et al. Integration of capecitabine (X) into adjuvant therapy comprising docetaxel (T) followed by 5-FU, epirubicin, and cyclophosphamide (CEF): efficacy in patients with triple-negative breast cancer (BC). Journal of Clinical Oncology 2010;28(15):Abstract no. 531.
-
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Asola R, et al. Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for breast cancer: an open-label, randomised controlled trial. Lancet Oncology 2009;10(12):1145-51. [DOI: 10.1016/S1470-2045(09)70307-9] - DOI - PubMed
-
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, et al. Abstract S4-1: FinXX Final 5-year analysis: results of the randomised, open-label, phase III trial in medium-to-high risk early breast cancer. Cancer Research 2010;70(24 Suppl):Abstract no. S4-1.
GEICAM 2003‐10 {published data only}
-
- Bermejo B, Ruiz, A, Borrego MR, Ribelles N, Rodriguez-Lescure A, Munoz-Mateu M, et al. Randomized phase III study of adjuvant chemotherapy for node-positive early breast cancer (BC) patients (pts) comparing epirubicin plus cyclophosphamide followed by docetaxel (EC-T) versus epirubicin plus docetaxel followed by capecitabine (ET-X): efficacy analysis of the GEICAM/2003-10 trial. Journal of Clinical Oncology 2013;31(15 Suppl):Abstract no. 1027.
-
- Martin M, Ruiz Simon A, Ruiz Borrego M, Ribelles N, Rodriguez-Lescure A, Munoz-Mateu M, et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 study. Journal of Clinical Oncology 2015;33(32):3788-95. - PubMed
GeparQuattro {published data only}
-
- Huober J, Loibl S, Untch M, RB-Esfahani S, Solbach C, Tesch H, et al. Abstract PD02-06: New molecular biomarkers for resistance to trastuzumab in primary HER2 positive breast cancer - a translational investigation from the neoadjuvant GeparQuattro study. Cancer Research 2010;70(24 Suppl):D02-6.
-
- Huober JB, Denkert C, Minckwitz G, Prinzler J, Kronenwett R, Darb-Esfahani Silvia, et al. Impact of expression levels of mRNA HER2 and ESR1 on the pathologic complete remission (pCR) rate after neoadjuvant treatment with anthracycline-taxane containing chemotherapy in combination with trastuzumab in the GeparQuattro trial. Journal of Clinical Oncology 2012;30(15 Suppl):Abstract no. 523.
-
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Abstract S1-05: Tumor infiltrating lymphocytes (TILs) indicate trastuzumab benefit in early-stage HER2-positive breast cancer (HER2+ BC). Cancer Research 2013;73(24 Suppl):Abstract no. S1-05. [DOI: 10.1158/0008-5472.sabcs13-s1-05] - DOI
-
- Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober, J, Tesch H, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. Journal of Clinical Oncology 2010;28(12):2015-23. [DOI: 10.1200/JCO.2009.23.8303] - DOI - PubMed
-
- Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clinical Cancer Research 2010;16(9):2634-45. - PubMed
ICE {published data only}
-
- Reimer T, Joel B, Minckwitz G, Potenberg J, Conrad B, Graf H, et al. Quality of life (QoL) in elderly patients (pts) with early-stage breast cancer treated with ibandronate (I) with or without capecitabine (X): results of the GBG 32 ICE trial. European Journal of Cancer Supplement 2009;7(2-3):219-20.
-
- Reimer T, Nitz U, Potenberg J, Conrad B, Graf H, Just M, et al. ICE Study: A prospective, multi-centre, controlled, open-label, randomized phase III trial of ibandronate (I) with or without capecitabine (X) in elderly patients (pts) with early breast cancer (GBG 32). European Journal of Cancer Supplements 2009;7(2):215. [DOI: 10.1016/S1359-6349(09)70737-2] - DOI
-
- Minckwitz G, Reimer T, Potenberg J, Conrad B, Schürer U, Eidtmann H, et al. Abstract S3-04: The phase III ICE study: adjuvant Ibandronate with or without capecitabine in elderly patients with moderate or high risk early breast cancer. Cancer Research 2015;75(9 Suppl):S3-4.
IMELDA {published data only}
-
- Doval D, Cinieri S, Bozcuk H, Pierga J-Y, Altundag K, Wang X, et al. Abstract P2-12-16: Exploratory post hoc analyses of patient-reported outcomes (PROs) in the IMELDA randomized phase III trial: maintenance bevacizumab (BEV) ± capecitabine (CAP) after initial first-line BEV plus docetaxel (DOC) for HER2-negative metastatic breast cancer. Cancer Research 2015;75(9 Suppl):P2-12.
-
- Gligorov J, Bines J, Alba E, Mustacchi G, Cinieri S, Gupta V, et al. Abstract P2-17-01: Overall survival (OS) in the IMELDA randomized phase III trial of maintenance bevacizumab (BEV) with or without capecitabine (CAP) for HER2-negative metastatic breast cancer (mBC). Cancer Research 2015;75(9 Suppl):P2-17.
-
- Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncology 2014;15(12):1351-60. - PubMed
-
- Gligorov J, Doval D, Bines J, Jiang Z, Alba E, Cortes P, et al. Efficacy and safety of maintenance bevacizumab (bev) with or without capecitabine (cap) after initial first-line bev plus docetaxel (doc) for HER2-negative metastatic breast cancer (mbc): IMELDA randomised phase III trial. Annals of Oncology 2014;25(4 Suppl):iv116-7. [DOI: 10.1093/annonc/mdu329.2] - DOI
-
- Mustacchi G, Bines J, Alba E, Cortes P, Doval D, De Ducla S, et al. Impact of post-progression therapy on overall survival (OS) in the IMELDA randomized phase III trial evaluating the addition of capecitabine (CAP) to maintenance bevacizumab (BEV) for HER2-negative metastatic breast cancer (mBC). Cancer Research 2017;77(4 Suppl):Abstract no. P5-15-06.
Lee 2008 {published data only}
-
- Ahn JB, Oh JH, Kwon Y, Chung KW, Kang HS, Shin KH, et al. Interim analysis findings from a phase III randomized trial of docetaxel/capecitabine (TX) vs. doxorubicin/cyclophosphamide (AC) as primary chemotherapy for stage II/III breast cancer (BC). Annals of Oncology 2004;15(3 Suppl):215PD.
-
- Jeong JH, Jung SY, Park IH, Lee KS, Kang HS, Kim SW, et al. Predictive factors of pathologic complete response and clinical tumor progression after preoperative chemotherapy in patients with stage II and III breast cancer. Investigational New Drugs 2012;30(1):408-16. [DOI: 10.1007/s10637-010-9555-7] - DOI - PubMed
-
- Lee HG, Lee JJ, Jung KH, Kwon Y, Shin EH, Chung KW, et al. Phase III randomized trial of primary chemotherapy with doxorubicin/cyclophosphamide (AC) vs docetaxel/capecitabine (TX) for stage II/III breast cancer (BC): interim analysis. Journal of Clinical Oncology 2004;22(14 Suppl):Abstract no. 607.
-
- Lee KS, Lee ES, Kwon YM, Nam BH, Kwon HS, Chung KW, et al. Mature results from a randomized phase III trial of docetaxel/capecitabine (TX) vs. doxorubicin/cyclophosphamide (AC) as primary chemotherapy for patients (pts) with stage II/III breast cancer (BC) [abstract no: 5052]. Breast Cancer Research & Treatment 2005;94(Suppl 1):S224.
-
- Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, Chung KW, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Research and Treatment 2008;109(3):481-9. [DOI: 10.1007/s10549-007-9672-y] - DOI - PubMed
METRIC {published data only}
-
- Schmid P, Melisko M, Yardley DA, Blackwell K, Forero A, Ouellette G, et al. METRIC: a randomized international study of the antibody drug conjugate (ADC) glembatumumab vedotin (GV, CDX-011) in patients (pts) with metastatic gpNMB overexpressing triple-negative breast cancer (TNBC). Annals of Oncology 2016;27(6 Suppl):VI97.
NSABP‐40 {published data only}
-
- Bear HD, Tang G, Rastogi P, Geyer CE, Andre R, Atkins JN, et al. The effect on surgical complications of bevacizumab added to neoadjuvant chemotherapy: NSABP protocol B-40. Cancer Research 2011;71(24 Suppl):Abstract no. PD07-08.
-
- Bear HD, Tang G, Rastogi P, Geyer CE, Liu Q, Robidoux A, et al. Abstract PD2-1: The effect on overall and disease-free survival (OS & DFS) by adding bevacizumab and/or antimetabolites to standard neoadjuvant chemotherapy: NSABP Protocol B-40. Cancer Research 2015;75(9 Suppl):PD2-1.
-
- Bear HD, Tang G, Rastogi P, Geyer CE, Robidoux A, Atkins JN, Baez L, et al. The effect on pCR of bevacizumab and/or antimetabolites added to standard neoadjuvant chemotherapy: NSABP protocol B-40. Journal of Clinical Oncology 2011;29(18 Suppl):Abstract no. LBA1005.
Pallis 2012 {published data only}
-
- Mavroudis D, Ardavanis A, Boukovinas I, Varthalitis I, Syrigos K, Potamianou A, et al. A multicenter randomized study comparing vinorelbine plus gemcitabine versus capecitabine monotherapy as salvage treatment in patients with advanced breast cancer pretreated with taxane and anthracycline chemotherapy: a preliminary report. Journal of Clinical Oncology 2006;24(18 Suppl):42s, abstract no. 658.
-
- Pallis AG, Boukovinas I, Ardavanis A, Varthalitis I, Malamos N, Georgoulias V, et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Annals of Oncology 2012;23(5):1164-9. - PubMed
Seidman 2011 {published data only}
-
- Seidman AD, Brufsky A, Ansari RH, Hart LL, Stein RS, Schwartzberg LS, et al. Phase III trial of gemcitabine plus docetaxel versus capecitabine plus docetaxel with planned crossover to the alternate single agent in metastatic breast cancer. Annals of Oncology 2011;22(5):1094-101. [DOI: 10.1093/annonc/mdq578] - DOI - PubMed
-
- Seidman AD, Brufsky A, Ansari RH, Rubinsak JR, Stein RS, Schwartzberg LS, et al. Phase III trial of gemcitabine plus docetaxel (GD) compared to capecitabine plus docetaxel (CD) with planned crossover to the alternate single agent in metastatic breast cancer (MBC). Journal of Clinical Oncology 2009;27(15):1000.
SO140999 {published data only}
-
- Gluck S, Russel C, O'Shaughnessy J, Yuan G, Odom D, Sherrill B, et al. Relationship between survival and estrogen receptor (ER) status in pts with metastatic breast cancer (MBC) treated with capecitabine (C) and docetaxel (D): an exploratory data analysis. Journal of Clinical Oncology 2009;27(15):1024.
-
- Gluck S, Russell C, O'Shaughnessy J, McKenna EF, Hu S, Odom D, et al. Treatment effect of capecitabine and docetaxel or docetaxel alone by oestrogen receptor status in patients with metastatic breast cancer: results of an exploratory analysis. Breast 2013;22(6):1087-93. [DOI: 10.1016/j.breast.2013.08.016] - DOI - PubMed
-
- Leonard R, Cervantes G, Lui W, Mauriac L, Miles D, Moiseyenko V, et al. Survival update of so14999 a large phase III trial of capecitabine/doxetaxel combination therapy vs docetaxel monotherapy in patients with locally advanced (LABC) or metastatic breast cancer (MBC). European Journal of Cancer 2001;37(6):S151. [DOI: 10.1016/S0959-8049(01)81043-1] - DOI
-
- Leonard R, Malinovszky L. Poorer survival of patients in UK and Russia in an international randomised trial of chemotherapy for metastatic breast cancer. European Journal of Cancer 2002;38(Suppl 3):S65-75; abstract no. 131.
-
- Leonard R, O'Shaughnessy J, Vukelja S, Gorbounova V, Chan-Navarro CA, Maraninchi D, et al. Detailed analysis of a randomized phase III trial: can the tolerability of capecitabine plus docetaxel be improved without compromising its survival advantage? Annals of Oncology 2006;17(9):1379-85. [DOI: 10.1093/annonc/mdl134] - DOI - PubMed
Study 301 {published data only}
-
- Awada A, Kaufman PA, Yelle L, Cortes J, Wanders J, O'Shaughnessy J, et al. A phase III, open-label, randomized study of eribulin versus capecitabine in patients (pts) with metastatic breast cancer (MBC): effect of post-progression anti-cancer treatments (PPT) and metastatic progression events on overall survival. Cancer Research 2013;73(24 Suppl):Abstract no. P3-13-03.
-
- Cortés J, Pérez J, He Y, Metzger-Filho O. Abstract P3-13-06: Efficacy of eribulin in patients with invasive lobular carcinoma of the breast: data from a pooled analysis. Cancer Research 2015;75(9 Suppl):Abstract no. P3-13-06.
-
- Cortes J, Awada A, Kaufman PA, Yelle L, Perez EA, Velikova G, et al. Quality of life (QoL) in patients (pts) with locally advanced or metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes who received eribulin mesylate or capecitabine: a phase III, open-label, randomized study. Journal of Clinical Oncology 2013;31(15 Suppl):Abstract no. 1050.
-
- Cortes J, Awada A, Twelves C, Yelle L, Wanders J, Olivo MS, et al. Quality of life in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes who received eribulin mesylate or capecitabine in a phase III, open-label, randomized study. Cancer Research 2012;72(24 Suppl):Abstract no. P1-12-08. [DOI: 10.1158/0008-5472.sabcs12-p1-12-08] - DOI
-
- Cortes J, Hudgens S, Twelves C, Perez EA, Awada A, Yelle L, et al. Health-related quality of life in patients with locally advanced or metastatic breast cancer treated with eribulin mesylate or capecitabine in an open-label randomized phase 3 trial. Breast Cancer Research and Treatment 2015;154(3):509-20. - PMC - PubMed
TABEA {published data only}
-
- Lueck HJ, Luebbe K, Bischoff J, Maass N, Feisel G, Tome O, et al. A randomized phase III study to determine the efficacy of capecitabine in addition to a taxane and bevacizumab as first-line therapy in patients with metastatic breast cancer. Journal of Clinical Oncology 2013;31(15 Suppl):Abstract no. 1082.
TACT2 {published data only}68068041
-
- Bayani J, Morden J, Skaria S, Bliss P, Grieve R, Harnett A, et al. Abstract P4-11-03: Androgen receptor expression is an independent marker of lower residual risk in the TACT2 trial (CRUK/05/019). Cancer Research 2015;75(9 Suppl):Abstract no. P4-11-03. [DOI: ]
-
- Bliss JM, Canney P, Velikova G, Barrett-Lee P, Moyses H, McDermaid M, et al. Abstract P5-10-07: TACT2 randomised adjuvant trial in early breast cancer (EBC): tolerability and toxicity of standard 3 weekly epirubicin (E) versus accelerated epirubicin (aE) followed by capecitabine (X) or CMF in 129 UK hospitals (CRUK/05/019) 771. Cancer Research 2010;70(24 Suppl):5-10.
-
- Cameron D, Barrett-Lee P, Canney P, Banerji J, Bartlett J, Bloomfield D, et al. Abstract S3-3: The UK TACT2 Trial: comparison of standard vs accelerated epirubicin in patients requiring chemotherapy for early breast cancer (EBC) (CRUK/05/019). Cancer Research 2012;72(24 Suppl):Abstract no. S3-3. [DOI: 10.1158/0008-5472.sabcs12-s3-3] - DOI
-
- Cameron D, Barrett-Lee P, Velikova G, Canney P, Moyses H, McDermaid M, et al. Abstract P5-10-06: TACT2 randomised adjuvant trial in early breast cancer (EBC): tolerability and toxicity of standard 3 weekly epirubicin (E) versus accelerated epirubicin (aE) in 129 UK hospitals (4391 patients) (CRUK/05/019). Cancer Research 2010;70(24 Suppl):Abstract no. P5-10-06.
-
- Cameron D, Morden JP, Canney P, Velikova G, Coleman R, Bartlett J, et al. Accelerated versus standard epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil or capecitabine as adjuvant therapy for breast cancer in the randomised UK TACT2 trial (CRUK/05/19): a multicentre, phase 3, open-label, randomised, controlled trial. Lancet Oncology 2017;18(7):929-45. - PMC - PubMed
TURANDOT {published data only}
-
- Beslija S, Brodowicz T, Greil R, Inbar MJ, Kahan Z, Kaufman B, et al. First-line bevacizumab in combination with capecitabine or paclitaxel for HER2-negative locally recurrent or metastatic breast cancer (LR/MBC): a randomized phase III trial. Cancer Research 2011;71(24 Suppl):Abstract no. OT2-01-02. - PubMed
-
- Brodowicz T, Pienkowski T, Beslija S, Melichar B, Lang I, Inbar MJ, et al. Abstract P6-06-40: Analysis of outcome according to risk factors in the randomized phase III TURANDOT trial evaluating first-line bevacizumab-containing therapy for HER2-negative locally recurrent/metastatic breast cancer (LR/mBC). Cancer Research 2013;73(24 Suppl):Abstract no. P6-06-40.
-
- Christoph ZC, Istvan L, Semir B, Zsuzsanna K, Inbar MJ, Salomon SM, et al. Hand-foot-syndrome (HFS) is a strong predictor for OS and PFS in HER2-negative metastatic breast cancer (mBC) treated with first-line capecitabine (CAP) + bevacizumab (BEV): results of a subanalysis of the randomized phase III CECOG TURANDOT trial. Cancer Research 2015;75(9 Suppl):Abstract no. P3-06-10.
-
- Inbar M, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, et al. Randomized phase III study of first-line bevacizumab in combination with capecitabine or paclitaxel for HER2-negative LR/MBC: interim safety data. European Journal of Cancer 2011;47(Suppl 1):S346. [DOI: 10.1016/S0959-8049(11)71495-2] - DOI - PubMed
USON 01062 {published data only}
-
- NCT00089479. A randomized, open-label, multicenter, phase III trial comparing regimen of adriamycin plus cytoxan followed by either taxotere or taxotere plus Xeloda as adjuvant therapy for female patients with high-risk breast cancer [A study of Xeloda (capecitabine) in women with high-risk breast cancer]. clinicaltrials.gov/ct2/show/NCT00089479 2004;(PDQ; NO17629; NCT00089479).
-
- O'Shaughnessy J, Koeppen H, Crockett M, Lackner M, Spoerke JM, Wilson T, et al. Abstract P6-09-01: Central Ki67 analysis as a predictor for adjuvant capecitabine efficacy in early breast cancer (EBC) subtypes in US oncology trial 01062. Cancer Research 2013;73(24 Suppl):Abstract no. P6-09-01.
-
- O'Shaughnessy J, Koeppen H, Xiao Y, Lackner MR, Paul D, Stokoe C, et al. Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clinical Cancer Research 2015;21(19):4305-11. - PubMed
-
- O'Shaughnessy J, Loesch DM, Paul D, Stokoe CT, Pippen JE, Blum JL, et al. ER as a predictor of early breast cancer (EBC) outcomes in patients. Journal of Clinical Oncology 2013;31(15 Suppl):Abstract no. 590.
-
- O'Shaughnessy J, Paul D, Stokoe C, Pippen Jr J, Blum JL, Krekow L, et al. Abstract S4-2: First efficacy results of a randomized, open-label, phase III study of adjuvant doxorubicin plus cyclophosphamide, followed by docetaxel with or without capecitabine, in high-risk early breast cancer 24 2672. Cancer Research 2010;70(24 Suppl):S4-2.
Yoo 2015 {published data only}
-
- Yoo C, Kim SB, Ahn JH, Kim JE, Jung KH, Gong GY, et al. A randomized phase II trial of capecitabine plus vinorelbine followed by docetaxel versus adriamycin plus cyclophosphamide followed by docetaxel as neoadjuvant chemotherapy for breast cancer. Cancer Research and Treatment 2014;47(3):406-15. - PMC - PubMed
Zhang 2016 {published data only}
-
- Zhang M, Wei W, Liu J, Yang H, Jiang Y, Tang W, et al. Comparison of the effectiveness and toxicity of neoadjuvant chemotherapy regimens, capecitabine/epirubicin/cyclophosphamide vs 5-fluorouracil/epirubicin/cyclophosphamide, followed by adjuvant, capecitabine/docetaxel vs docetaxel, in patients with operable breast cancer. OncoTargets and Therapy 2016;9:3443-50. - PMC - PubMed
References to studies excluded from this review
ACTRN12613000206729 {published data only}
-
- ACTRN12613000206729. A randomized, multi-centre, open-label study: comparison of the efficacy and safety of neo-adjuvant taxanes and capecitabine(TX) versus taxanes and anthracycline(TA) in patients with breast cancer. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362825&isReview... (first received 16 February 2013).
AHX‐03‐202 {published data only}
-
- Rivera E, Chang JC, Semiglazov V, Gorbunova V, Manikhas A, Krasnozhon D, et al. Eniluracil + 5-fluorouracil + leucovorin (EFL) vs. capecitabine. Phase 2 trial for metastatic breast cancer (AHX-03-202). Cancer Research 2012;72(24 Suppl):OT3-3-01. [DOI: 10.1158/0008-5472.sabcs12-ot3-3-01] - DOI
ANZ 0001 {published data only}
-
- Stockler M, Sourjina T, Grimison P, Gebski V, Byrne M, Harvey V, et al. A randomized trial of capecitabine (C) given intermittently (IC) rather than continuously (CC) compared to classical CMF as first-line chemotherapy for advanced breast cancer (ABC). Journal of Clinical Oncology 2007;25(18 Suppl):Abstract no. 1031.
-
- Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. Journal of Clinical Oncology 2011;29(34):4498-504. [DOI: 10.1200/JCO.2010.33.9101] - DOI - PubMed
Berton Rigaud 2008 {published data only}
-
- Berton-Rigaud D, Roche H, Penault-Llorca F, Tubiana-Mathieu N, Ferrero J, Coudert B, et al. Benefit of neoadjuvant capecitabine + epirubicin + cyclophosphamide (CEX) versus 5-FU + epirubicin + cyclophosphamide (FEC) for operable breast cancer (BC) followed by adjuvant docetaxel (T). Journal of Clinical Oncology 2008;26(15 Suppl):Abstract no. 598.
Beslija 2006 {published data only}
-
- Beslija S, Obralic N, Basic H, Tatarevic A, Mahic N, Banjin M. A single institution randomized trial of taxotere (T) and xeloda (X) given in combination vs. taxotere (t) followed by xeloda (x) after progression as first line chemotherapy (CT) for metastatic breast cancer (MBC). European Journal of Cancer 2005;114(3 Suppl):Abstract 407.
-
- Beslija S, Obralic N, Basic H, Tatarevic A, Naila M, Banjin M, et al. Randomized trial of sequence vs. combination of capecitabine (X) and docetaxel (T): XT vs. T followed by X after progression as first-line therapy for patients (pts) with metastatic breast cancer (MBC). Journal of Clinical Oncology 2006;24(18 Suppl):Abstract no. 571.
CALBG 49907 {published data only}
-
- Freedman RA, Pitcher B, Keating NL, Ballman KV, Mandelblatt J, Kornblith AB, et al. Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Research & Treatment 2013;139(2):607-16. [DOI: 10.1007/s10549-013-2562-6] - DOI - PMC - PubMed
-
- Freedman RA, Pitcher B, Keating NL, Barry WT, Ballman KV, Kornblith A, et al. Changes in cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine within CALGB 49907. Cancer Research 2012;72(24):P6-08-05. [DOI: 10.1158/0008-5472.sabcs12-p6-08-05] - DOI - PMC - PubMed
-
- Gajra A, McCall L, Muss HB, Cohen HJ, Jatoi A, Ballman KV, et al. Abstract P5-15-07: Association of patient preference for adjuvant chemotherapy (chemo) at baseline (BL) with toxicity, mental health, function, quality of life (QoL) and survival in older women with early stage breast cancer (ESBC) [CALGB 49907 Alliance]. Cancer Research 2015;75(9 Suppl):Abstract no. P5-15-07.
Campone 2009 {published data only}
-
- Campone M, Dobrovolskaya N, Tjulandin S, Chen SC, Fourié SJ, Mefti F, et al. A 3-arm randomised phase II study of oral vinorelbine (NVBo) plus capecitabine (X) versus NVBo and X in sequential versus docetaxel (D) plus X in patients with metastatic breast cancer (MBC) previously treated with anthracyclines. EJC Supplements 2009;7(2):259. [DOI: 10.1016/S1359-6349(09)70892-4] - DOI - PubMed
ECTO‐II {published data only}2004‐004957‐2496423607
-
- Zambetti M, Mansutti M, Gomez P, Lluch A, Dittrich C, Zamagni C, et al. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel, randomized phase II trials with an adaptive study design (ECTO II) 134 33949. Breast Cancer Research & Treatment 2012;132(3):843-51. - PubMed
-
- Zambetti M, Mansutti M, Llunch A, Zamagni C, De Benedictis E, Gomez P, et al. First report of the European Cooperative Trial in Operable Breast Cancer II (ECTO II): effects of primary chemo-endocrine therapy on local-regional disease in ER-positive breast cancer. Journal of Clinical Oncology 2008;26(15 Suppl):Abstract no. 588.
-
- Zambetti M, Mansutti M, Llunch A, Zamagni C, De Benedictis E, Gomez P, et al. First report of the European Cooperative Trial in Operable Breast Cancer II (ECTO II): effects of primary chemo-endocrine therapy on local-regional disease in ER-positive breast cancer [abstract no. 588]. Journal of Clinical Oncology 2008;26(15 Suppl):28.
-
- Zambetti M, Pascoletti G, Gomez P, Semiglazov V, Lluch A, De Benedictis E, et al. European cooperative trial in operable breast cancer II (ECTO II): activity of primary chemotherapy in ER negative early breast cancer. European Journal of Cancer Supplements 2009;7(2):302.
EMBRACE {published data only}
-
- Blum JL, Twelves CJ, Akerele C, Seegobin S, Wanders J, Cortes J. Abstract P6-13-01: Impact of the number of prior chemotherapy regimens on overall survival (OS) among subjects with locally recurrent or metastatic breast cancer treated with eribulin mesylate: results from the Phase III EMBRACE study 941. Cancer Research 2010;70(24 Suppl):6-13.
-
- Cardoso F, Twelves C, Vahdat LT, Dutcus C, Seegobin S, Wanders J, Cortes J, et al. Eribulin mesylate EMBRACE study - Survival analysis excluding patients re-challenged with therapies of the same class. European Journal of Cancer 2011;47:S331-2.
-
- Cortes J, Awada A, Kaufman PA, Yelle L, Perez EA, Velikova G, et al. Quality of life (QoL) in patients (pts) with locally advanced or metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes who received eribulin mesylate or capecitabine: a phase III, open-label, randomized study. Journal of Clinical Oncology 2013;31(15 Suppl):Abstract no. 1050.
-
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011;377(9769):914-23. - PubMed
-
- Cortes J, Twelves C, Wanders J, Wang W, Vahdat L, Dutcus C. Clinical response to eribulin in patients with metastatic breast cancer is independent of time to first metastatic event. EMBRACE Study Group. Breast 2011;20(4 Suppl):S48-9. [DOI: 10.1016/j.breast.2011.08.106] - DOI
EORTC 10001 {published data only}
-
- NCT00049660. Phase II/III randomized study of capecitabine versus vinorelbine in women with metastatic breast cancer previously treated with taxanes with or without anthracyclines. clinicaltrials.gov/ct2/show/NCT00049660 (first received 27 January 2003);(PDQ; EORTC-10001; EORTC-16001O; IDBBC-EORTC-10001).
-
- Pajk B, Cufer T, Canney P, Ellis P, Cameron D, Blot E, et al. Anti-tumor activity of capecitabine and vinorelbine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: findings from the EORTC 10001 randomized phase II trial. Breast 2008;17(2):180-5. [DOI: 10.1016/j.breast.2007.09.002] - DOI - PubMed
ERASME‐4 {published data only}
-
- Bachelot T, Bajard A, Ray-Coquard I, Provencal J, Coeffic D, Agostini C, et al. Final results of ERASME-4: a randomized trial of first-line docetaxel plus either capecitabine or epirubicin for metastatic breast cancer 1032 34617. Oncology (Williston Park) 2011;80(3-4):262-8. - PubMed
-
- Bachelot T, Bajard A, Ray-Coquard I, Provencal J, Coeffic D, Dramais D, et al. Superiority of docetaxel + capecitabine compared to docetaxel + epirubicin as first-line therapy for metastatic breast cancer - final results of the ERASME-4 Study 881. Cancer Research 2009;69(24 Suppl):2099.
-
- Bachelot T, Bajard A, Ray-Coquard I, Provencal J, Coeffic D, Dramais D. Superiority of docetaxel + capecitabine compared to docetaxel + epirubicin as first-line therapy for metastatic breast cancer - final results of the ERASME-4 study. Cancer Research 2010;69(24 Suppl):Abstract no. 2099. [DOI: 10.1158/0008-5472.SABCS-09-2099] - DOI
-
- Bachelot T, Luporsi E, Bajard A, Provencal J, Coefic D, Platini C, et al. Randomized trial of first-line docetaxel + capecitabine (XT) versus docetaxel + epirubicin (ET) for metastatic breast cancer (MBC): efficacy results of ERASME-4/CAPEDOC-EPIDOC [abstract no. 1049]. Journal of Clinical Oncology 2008;26(15 Suppl):Abstract no. 1049.
-
- Luporsi E, Bachelot T, Bajard A, Provencal Chinal J, Coeffic D, Platini C, et al. Comparative efficacy of first-line docetaxel + capecitabine (XT) versus docetaxel + epirubicin (ET): pooled analysis of two randomised trials. Annals of Oncology 2008;19(S8):viii67-8.
Eremin 2015 {published data only}
-
- Eremin J, Cowley G, Walker LG, Murray E, Stovickova M, Eremin O. Women with large (≥3 cm) and locally advanced breast cancers (T3, 4, N1, 2, M0) receiving neoadjuvant chemotherapy (NAC: cyclophosphamide, doxorubicin, docetaxel): addition of capecitabine improves 4-year disease-free survival. SpringerPlus 2015;4(1):9. - PMC - PubMed
EudraCT 2010‐022646‐24 {published data only}
-
- EudraCT 2010-022646-24. Evaluation of masitinib combined to gemcitabine, carboplatin or capecitabine in patients with a metastatic or locally advanced breast cancer relapsing after a first chemotherapy treatment. www.clinicaltrialsregister.eu/ctr-search/trial/2010-022646-24/ES (first received 17 December 2014).
GAIN {published data only}
-
- Furlanetto J, Eiermann W, Marme F, Reimer T, Reinisch M, Schmatloch S, et al. Higher rate of severe toxicities in obese patients receiving dose-dense chemotherapy according to unadjusted body mass index - results of the prospectively randomized GAIN study. Cancer Research 2016;76(4 Suppl):Abstract no.P1-13-04. [DOI: ] - PubMed
-
- Hossain F, Peng Y, Pannuti A, Backus K, Golde T, Osborne B, et al. A novel non-canonical Notch1-IKK-mTORC2-AKT pathway maintains survival in triple negative breast cancer cells and cancer stem-like cells. Cancer Research 2017;77(4):Abstract no. P5-07-06.
-
- Moebus VJ, Von Minckwitz G, Jackisch C, Lueck HJ, Schneeweiss A, Tesch H, et al. German Adjuvant Intergroup Node Positive (GAIN) study: a phase III trial to compare IDD-ETC versus EC-TX in patients with node-positive primary breast cancer - final efficacy analysis. Journal of Clinical Oncology 2014;32(15):Abstract no. 1009.
-
- Van Rossum AGJ, Schouten PC, Weber KE, Nekljudova V, Denkert C, Von Minckwitz G, et al. BRCA1-like profile as predictive biomarker in non myeloablative chemotherapy (GAIN study). Cancer Research 2016;76(4 Suppl):Abstract no. P3-07-28. [DOI: ]
Gemcitabin 02 MC {published data only}
-
- Freier W, Stemmler HJ, Tessen H, Gitsch G, Jonat W, Brugger W, et al. Randomised trial comparing gemcitabine plus vinorelbine (Gem/Vin), gemcitabine plus cisplatin (Gem/Cis), and gemcitabine plus capecitabine (Gem/Cap) in pretreated metastatic breast cancer (MBC). Journal of Clinical Oncology 2008;26(15 Suppl):1054.
-
- Rozner R, Seidman AD. Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer. (Stemmler HJ, diGioia D, Freier W, et al. Ludwig-Maximilians Univ of Munich, Campus Grosshadern, Germany; Oncological Practice, Hildesheim, Germany; et al. British Journal of Cancer 2011;104:1071-8. Breast Diseases 2011;22(4):413-5. [DOI: 10.1016/j.breastdis.2011.10.014] - DOI - PMC - PubMed
-
- Stemmler HJ, diGioia D, Freier W, Tessen HW, Gitsch G, Jonat W, et al. Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer 32880 32880. British Journal of Cancer 2011;104(7):1071-8. [DOI: 10.1038/bjc.2011.86] - DOI - PMC - PubMed
Genta Incorporated 2012 {published data only}
-
- NCT01609127. Tesetaxel every 3 weeks vs weekly vs capecitabine as 1st-line therapy for locally advanced or metastatic breast cancer. www.clinicaltrials.gov/ct2/show/NCT01609127 (first received 31 May 2012).
Georgia CORE {published data only}
-
- Zelnak AB, Harichand-Herdt S, Styblo TM, Rizzo M, Gabram SGA, Bumpers HL, et al. Final results from randomized phase II trial of preoperative docetaxel (D) and capecitabine (C) given sequentially or concurrently for HER2-negative breast cancers. Journal of Clinical Oncology 2011;29(15 Suppl):1118.
-
- Zelnak AB, Styblo TM, Rizzo M, Gabram SG, Wood WC, Harichand-Herdt S, et al. Final results from phase II trial of neoadjuvant docetaxel and capecitabine given sequentially or concurrently for HER2-negative breast cancers. Clinical Breast Cancer 2013;13(3):173-9. [DOI: 10.1016/j.clbc.2012.12.004] - DOI - PubMed
GEPARTRIO {published data only}
-
- Costa S, Minckwitz G, Jackisch C, Raab G, Blohmer JU, Loehr A, et al. TAC as neoadjuvant chemotherapy in patients with primary breast cancer - interim progress report on 907 cases of the randomized prospective Gepartrio-trial. ASCO Meeting Abstracts 2004;22(14 Suppl):825.
-
- Costa SD, Loibl S, Kaufmann M, Zahm DM, Hilfrich J, Huober J, et al. Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. Journal of Clinical Oncology 2010;28(1):83-91. [DOI: 10.1200/JCO.2009.23.5101] - DOI - PubMed
-
- Costa SD, Loibl S, Kaufmann M, Zahm DM, Hilfrich J, Huober J, et al. Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. Journal of Clinical Oncology 2010;28(1):83-91. [DOI: 10.1200/JCO.2009.23.5101] - DOI - PubMed
-
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of Clinical Oncology 2010;28(1):105-13. - PubMed
-
- Gerber B, Minckwitz Blohmer JU, Loehr A, Raab G, Eidtmann H, Hilfrich J, et al. Effectiveness of vinorelbine/capecitabine (NX) versus docetaxel/doxorubicin/cyclophosphamide (TAC) in patients non-responding to 2 cycles of neoadjuvant TAC chemotherapy: first results of the phase III GEPARTRIO study by the German Breast Group. EJC Supplements 2006;4(2):148-9. [DOI: 10.1016/S1359-6349(06)80367-8] - DOI
Ghosn 2009 {published data only}
-
- Ghosn M, Aftimos P, Farhat FS, Kattan JG, Hanna C, Haddad N, et al. A phase II randomized study comparing navelbine and capecitabine (Navcap) followed either by Navcap or by weekly docetaxel in the first-line treatment of HER-2/neu negative metastatic breast cancer. Medical Oncology (Northwood, London, England) 2011;28(Suppl 1):S142-51. [DOI: 10.1007/s12032-010-9754-2] - DOI - PubMed
-
- Ghosn M, Farhat FS, Kattan JG, Hanna C, Younes F, Haddad N, et al. Navcap (vinorelbine and capecitabine) versus navcap followed by weekly docetaxel as first-line treatment in metastatic breast cancer patients: a randomized multicenter phase II trial. Journal of Clinical Oncology 2008;26(15 Suppl):1119.
-
- Ghosn M, Farhat FS, Kattan JG, Hanna C, Younes F, Haddad N, et al. Randomized multicenter phase II trial of Navcap (vinorelbine and capecitabine) versus Navcap followed by weekly docetaxel as first line treatment in Her-2/neu negative metastatic breast cancer patients: updated results. Cancer Research 2009;69(2 Suppl):Abstract no. 6116.
Giacchetti 2011 {published data only}
-
- Giacchetti S, Hajage D, Pierga JY, Delaloge S, Brain E, Tembo O, et al. Adjuvant chemotherapy with vinorelbine+5FU or capecitabine in poor responders to neoadjuvant EC-docetaxel chemotherapy (NAC) for locally advanced breast cancers. Cancer Research 2011;71(24 Suppl):P5-18-13.
GLICO‐0801 {published data only}
-
- Gomez H, Neciosup S, Tosello C, Mano M, Bines J, Ismael G, et al. Abstract P4-12-26: A phase II randomized study of lapatinib in combination with capecitabine, vinorelbine or gemcitabine as first or second line-therapy in patients with HER2 positive metastatic breast cancer progressing after taxane (LACOG 0801). Cancer Research 2013;73(24 Suppl):P4-12-26. [DOI: 10.1158/0008-5472.sabcs13-p4-12-26] - DOI
-
- Gomez HL, Neciosup SP, Neron Do Nascimento Y, Ismael G, Barrios H. A randomized open-label, phase II study of lapatinib/capecitabine, lapatinib/vinorelbine, or lapatinib/gemcitabine in patients (pts) with ErbB2-amplified metastatic breast cancer (MBC) progressing after taxane treatment-GLICO-0801. Journal of Clinical Oncology 2010;28(15 Suppl):TPS120.
-
- Gomez HL, Neciosup SP, Tosello C, Xavier P, Do Nascimento YN, Fanelli M, et al. A randomized, open-label, phase II study of lapatinib / capecitabine, lapatinib / vinorelbine, or lapatinib / gemcitabine in patients with ErbB2-amplified metastatic breast cancer progressing after taxane treatment: results of an interim analysis (GLICO-0801 / EGF111792). Journal of Clinical Oncology 2012;30(15 Suppl):e11087.
-
- NCT01050322. A randomized open-label, phase II study of lapatinib-capecitabine or lapatinib-vinorelbine or lapatinib/gemcitabine in subjects with Her2/Neu amplified metastatic breast cancer patients progression after taxanes treatment. clinicaltrials.gov/ct2/show/NCT01050322 (first received 15 January 2010).
Gruppo {published data only}
-
- Vici P, Giotta F, Di Lauro L, Sergi D, Vizza E, Mariani L, et al. A multicenter phase II randomized trial of docetaxel/gemcitabine versus docetaxel/capecitabine as first-line treatment for advanced breast cancer: a gruppo oncologico italia meridionale study. Oncology (Switzerland) 2011;81(3-4):230-6. [DOI: 10.1159/000334432] - DOI - PubMed
HellenicOncologyResearchGroup 2007 {published data only}
-
- NCT00440622. Study of gemcitabine and herceptin versus Xeloda and Herceptin in HER2 (+) metastatic breast cancer patients. www.clinicaltrials.gov/ct2/show/NCT00440622 (first received 27 February 2007).
HenriRoche 2006 {published data only}
-
- Roche H, Deporte R, Berton-Rigaud D, Coudert B, Tubiana-Mathieu N, Ferrero JM, et al. Updated safety findings from a randomized phase II trial of capecitabine + epirubicin + cyclophosphamide (cex) vs. 5-fu + epirubicin + cyclophosphamide (fec) as neoadjuvant therapy in patients (pts) with operable breast cancer (bc). Annals of Oncology 2006;17(9 Suppl):261P. [DOI: 10.1093/annonc/mdl206] - DOI
Hoffman 2004 {published data only}
-
- NCT00077857. Capecitabine in combo with intravenous docetaxel (Q3W) in patients with locally advanced and/or metastatic breast cancer. www.clinicaltrials.gov/ct2/show/NCT00077857 (first received 16 February 2004).
Hoffmann LaRoche 2015 {published data only}
-
- NCT01702558. Phase I study of the combination of trastuzumab emtansine (T-DM1) and capecitabine in HER2-positive metastatic breast cancer and HER2-positive locally advanced/metastatic gastric cancer patients, followed by a randomized, open-label phase II study of trastuzumab emtansine and capecitabine versus trastuzumab emtansine alone in HER2-positive metastatic breast cancer. clinicaltrials.gov/ct2/show/NCT01702558 (first received 8 October 2012).
HORG CT/02.09 {published data only}
-
- Marvoudis D, Papakotoulas P, Ardavanis A, Kakolyris S, Kouroussis C, Malamos N, et al. Docetaxel plus epirubicin versus docetaxel plus capecitabine as first line treatment in patients with advanced breast cancer. Final results of a multicenter phase III trial [Abstract No. 1360]. Annals of Oncology 2008;19(Suppl 8):viii63-4. - PubMed
-
- Marvoudis D, Papakotoulas P, Ardavanis A, Kakolyris S, Kouroussis C, Malamos N, et al. Docetaxel plus epirubicin versus docetaxel plus capecitabine as first line treatment in patients with advanced breast cancer. final results of a multicenter phase III trial [Abstract No. 136O]. Annals of Oncology 2009;19(Suppl 8):63-4. - PubMed
-
- Mavroudis D, Boukovinas I, Ardavanis A, Syrigos K, Kouroussis CH, Kakolyris S, et al. A multicenter phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first line treatment in patients with locally advanced and metastatic breast cancer. Preliminary report [abstract no: 6089] 1995. Breast Cancer Research & Treatment 2005;94(Suppl 1):S280.
-
- Mavroudis D, Papakotoulas P, Ardavanis A, Syrigos K, Kakolyris S, Ziras N, et al. Randomized phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first-line treatment in women with advanced breast cancer. Annals of Oncology 2010;21(1):48-54. [DOI: 10.1093/annonc/mdp498] - DOI - PubMed
Hu 2010 {published data only}
Hudis 2011 {published data only}
-
- Hudis C, Tauer KW, Hermann RC, Makari-Judson G, Isaacs C, Beck JT, et al. Sorafenib (SOR) plus chemotherapy (CRx) for patients (pts) with advanced (adv) breast cancer (BC) previously treated with bevacizumab (BEV). Journal of Clinical Oncology 2011;29(15 Suppl):Abstract no. 1009.
-
- Hudis CA, Baselga J, Gradishar WJ, Tauer KW, Hermann RC, Gomez P, et al. Sorafenib (SOR) plus chemotherapy (CRX) in patients (PTS) with triple-negative (TN) advanced (ADV) breast cancer (BC): Subgroup analyses of 3, double-blind, randomised, placebo (PL)-controlled phase 2B trials from the ties (trials to investigate the efficacy of sorafenib) bc program. Annals of Oncology 2011;22:ii54.
-
- Hudis CA, Hermann RC, Makari-Judson G, Isaacs C, Beck JT, Kaklamani VG, et al. Sorafenib (SOR) plus chemotherapy (CRx) for treatment (tx) of patients (pts) with HER2-negative locally advanced (adv) or metastatic (met) breast cancer (BC) and prior bevacizumab (BEV): subgroup analysis of AC01B07. European Journal of Cancer 2011;47(Suppl 1):S338.
ICE‐II {published data only}
-
- Von Minckwitz G, Conrad B, Decker T, Reimer T, Hackmann J, Potenberg J, et al. Final results from a randomised phase II study comparing epirubicin plus cyclophosphamide (EC) or CMF versus nab-paclitaxel plus capecitabine (PX) as adjuvant chemotherapy for elderly non-frail breast cancer patients with an increased risk of relapse. European Journal of Cancer 2014;50(Suppl 2):S109.
-
- Minckwitz G, Conrad B, Reimer T, Decker T, Eidtmann H, Eiermann W, et al. A randomized phase 2 study comparing EC or CMF versus nab-paclitaxel plus capecitabine as adjuvant chemotherapy for nonfrail elderly patients with moderate to high-risk early breast cancer (ICE II-GBG 52). Cancer 2015;121(20):3639-48. - PubMed
-
- Von Minckwitz G. ICE II: An investigational randomized phase II study on epirubicin (E) plus cyclophosphamide (C) (or CMF) versus nab-paclitaxel plus capecitabine (PX) as adjuvant chemotherapy for elderly nonfrail patients with an increased risk for relapse of a primary carcinoma of the breast. Journal of Clinical Oncology 2010;28(15 Suppl):TPS104.
ID01‐580 {published data only}
-
- Buzdar AU, Green MC, Broglio KR, Carter CD, Valero V, Ibrahim NK, et al. Prospective randomized trial evaluating weekly paclitaxel (WP) versus docetaxel in combination with capecitabine (DC) in operable breast cancer. Journal of Clinical Oncology 2009;27(15 Suppl):542. - PubMed
-
- Kelly CM, Green MC, Broglio K, Pusztai L, Thomas E, Brewster A, et al. Capecitabine in operable triple receptor-negative breast cancer: A subgroup analysis of a randomized phase III trial. Journal of Clinical Oncology 2011;29(27 Suppl):292.
Istituto Europeo di Oncologia 2006 {published data only}
-
- EUCTR2005-005046-39-IT. A randomized phase II study to assess the activity and tolerability of two regimens of metronomic oral chemotherapy methotrexate plus cyclophosphamide and cyclophosphamide plus capecitabine combined with bevacizumab in advanced breast cancer. clinicaltrialsregister.eu/ctr-search/trial/2005-005046-39/IT (date first received 7 April 2006.
JBCRN 05 {published data only}
-
- Yamamoto D, Iwase S, Odagiri H, Kuroda Y, Akazawa K, Kitamura K, et al. A randomized multicenter phase II trial of capecitabine versus S-1 as first-line treatment in unresectable or recurrent breast cancer patients. Journal of Clinical Oncology 2010;28(15 Suppl):TPS136.
-
- Yamamoto D, Iwase S, Tsubota Y, Ariyoshi K, Kawaguchi T, Miyaji T, et al. Randomized study of orally administered fluorinated pyrimidines (capecitabine versus S-1) in women with metastatic or recurrent breast cancer: Japan Breast Cancer Research Network 05 Trial. Cancer Chemotherapy & Pharmacology 2015;75(6):1183-9. [DOI: ] - PubMed
-
- Yamamoto D, Iwase S, Yamamoto C, Tsubota Y, Yamaguchi T. Randomized study of capecitabine versus S-1 in women with metastatic or recurrent breast cancer: Japan Breast Cancer Research Network (JBCRN) 05 trial. Journal of Clinical Oncology 2013;31(15 Suppl):1111.
Kourlaba 2014 {published data only}
-
- Kourlaba G, Rapti V, Alexopoulos A, Relakis J, Koumakis G, Chatzikou M, et al. Cost effectiveness analysis of everolimus plus exemestane vs. bevacizumab plus paclitaxel and bevacizumab plus capecitabine for the management of postmenopausal women with ER+ breast cancer. Annals of Oncology 2014;25(Suppl 4):IV117. [DOI: 10.1093/annonc/mdu329.4] - DOI
-
- Kourlaba G, Rapti V, Alexopoulos A, Relakis J, Koumakis G, Chatzikou M, et al. Economic evaluation of everolimus plus exemestane versus bevacizumab plus paclitaxel and bevacizumab plus capecitabine for the management of postmenopausal women with ER+ breast cancer. Journal of Clinical Oncology 2014;32(15 Suppl):e17638.
Lam {published data only}
-
- Lam SW, Groot SM, Honkoop AH, Jager A, ten Tije AJ, Bos MM, et al. Paclitaxel and bevacizumab with or without capecitabine as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a multicentre, open-label, randomised phase 2 trial. European Journal of Cancer 2014;50(18):3077-88. - PubMed
-
- Lam SW, De Groot SM, Honkoop AH, Jager A, Ten Tije AJ, Bos MMEM, et al. Combination of paclitaxel and bevacizumab without or with capecitabine as first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (LR/MBC): First results from a randomized, multicenter, open-label, phase II study of the Dutch Breast Cancer Trialists' Group (BOOG). Cancer Research 2011;71(24 Suppl):Abstract no PD07-07.
-
- Lam SW, Groot SM, Honkoop AH, Nota NM, Jager A, Velden AMT, et al. Plasma VEGF-a, angiopoietin-2 (ANG2) and soluble(s)TIE2 in patients (pts) with HER2-negative locally recurrent or metastatic breast cancer (LR/MBC) treated with first-line bevacizumab (A) and paclitaxel (T) without or with capecitabine (X). Journal of Clinical Oncology 2013;31(15 Suppl):1072.
-
- Lam SW, Frederiks CN, Van Der Straaten T, De Groot SM, Jager A, Bos MMEM, et al. Genetic polymorphisms (SNPs) as predictive markers for paclitaxel-induced peripheral neuropathy (PNP) and capecitabine-induced hand-foot syndrome (HFS) in HER-2 negative metastatic breast cancer patients. Cancer Research 2015;75(9 Suppl):Abstract no. P6-08-09.
LiNanlin 2013 {published data only}
-
- NCT02012634. Metronomic chemotherapy of capecitabine after standard adjuvant chemotherapy in operable triple negative breast cancer (MACRO). clinicaltrials.gov/ct2/show/NCT02012634 (first received 16 December 2013).
Lindner 2015 {published data only}
-
- Lindner JL, Loibl S, Denkert C, Ataseven B, Fasching PA, Pfitzner BM, et al. Expression of secreted protein acidic and rich in cysteine (SPARC) in breast cancer and response to neoadjuvant chemotherapy. Annals of Oncology 2015;26(1):95-100. - PubMed
Loman 2016 {published data only}
-
- Loman N, Linderholm B, Joensuu H, Ejlertsen B, Johannsson OT, Geisler J, et al. Nordic trip, a randomized phase 3 study in early triple negative breast cancer. Cancer Research 2016;76(4 Suppl):Abstract no OT3-02-10. [DOI: ]
MAMMA‐3 {published data only}
-
- Lück HJ, du Bois A, Loibl S, Schrader I, Huober J, Heilmann V, et al. Capecitabine plus paclitaxel versus epirubicin plus paclitaxel as first-line treatment for metastatic breast cancer: efficacy and safety results of a randomized, phase III trial by the AGO Breast Cancer Study Group. Breast Cancer Research & Treatment 2013;139(3):779-87. - PubMed
-
- Lück HJ, du Bois A, Schrader I, Huober J, Heilmann V, Fasching PA, et al. Final results of the AGO breast cancer study group MAMMA-3 trial: first-line capecitabine + paclitaxel vs epirubicin + paclitaxel for high-risk metastatic breast cancer. Breast Cancer Research and Treatment 2007;106:S67. - PubMed
-
- Lueck H, Minckwitz GV, Du Bois A, Schrader I, Huober J, Heilmann V, et al. Epirubicin/paclitaxel (EP) vs. Capecitabine/Paclitaxel (XP) in first-line metastatic breast cancer (MBC): A prospective, randomized multicentre phase III study of the AGO breast cancer study group. Journal of Clinical Oncology 2006;24(18 Suppl):517.
Mansutti 2008 {published data only}
-
- Mansutti M, Cavazzini G, Lorusso V, Boni C, Ocelli M, Puglisi F, et al. Randomized, multicenter, phase III trial of docetaxel plus epirubicin (ET) with or without capecitabine (X) as first-line therapy for stage IV breast cancer (BC). Journal of Clinical Oncology: ASCO.annual meeting proceedings 2008;26(15 Suppl):Abstract no 1034.
-
- Mansutti M, Gianpiero F, Cavazzini G, Durando A, Russo VL, Nardi M, et al. Randomised phase III trial comparing TEX (docetaxel, epirubicin and capecitabine) vs. TE (docetaxel and cpirubicin) in advanced breast cancer patients: findings from the 2nd interim analysis. Annals of Oncology 2004;15(3 Suppl):157P.
Martin 2015 {published data only}
-
- Martin M, Beslija S, Carrasco E, Kahan Z, Escudero MJ, Láng I, et al. 409TiPPhase III study of palbociclib in combination with exemestane vs. capecitabine, in hormone receptor (HR) positive/HER2 negative metastatic breast cancer (mbc) patients with resistance to non-steroidal aromatase inhibitors (NSAI): Pearl study (GEICAM/2013-02_CECOG/BC.1.3.006). Annals of Oncology 2014;25(4 Suppl):iv134-5. [DOI: 10.1093/annonc/mdu329.59] - DOI
-
- Martin M, Beslija S, Carrasco E, Kahan Z, Escudero MJ, Lang I, et al. Phase III study of palbociclib in combination with exemestane vs. capecitabine, in hormonal receptor (HR) positive/HER2 negative metastatic breast cancer (MBC) patients with resistance to non-steroidal aromatase inhibitors (NSAI): PEARL study (GEICAM/2013-02-CECOG/BC.1.3.006). Cancer Research 2015;75(9 Suppl):OT1-1-05. [DOI: ]
-
- Martin M, Inbar MJ, Carrasco EM, Lang I, Escudero MJ, Kahan Z, et al. Phase III study of palbociclib in combination with exemestane vs. capecitabine, in hormonal receptor (HR) positive/HER2 negative metastatic breast cancer (MBC) patients with resistance to non-steroidal aromatase inhibitors (NSAI): PEARL study (GEICAM/2013-02-CECOG/BC.1.3.006). Journal of Clinical Oncology 2015;33(15 Suppl):TPS631.
Matter‐Walstra 2015 {published data only}
-
- Matter-Walstra KW, Bigler M, Schwenkglenks M, Bertschi D, Brechbühl J, Hasler-Strub U, et al. Abstract P1-10-06: Health economic evaluation of: Bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced breast cancer: A multicenter, randomized phase III trial - SAKK 24/0. Cancer Research 2015;75(9 Suppl):Abstract no P1-10-06.
Mavroudis 2006 {published data only}
-
- Mavroudis D, Ardavanis A, Boukovinas I, Varthalitis I. A multicenter randomized study comparing vinorelbine plus gemcitabine versus capecitabine monotherapy as salvage treatment in patients with advanced breast cancer pretreated with taxane and anthracycline chemotherapy: A preliminary report. Journal of Clinical Oncology 2006;24(18 Suppl):658.
Melisko 2016 {published data only}
-
- Melisko M, Yardley DA, Blackwell K, Forero A, Ma C, Montero A, et al. Abstract OT1-03-15: The METRIC trial: A randomized international study of the antibody-drug conjugate glembatumumab vedotin (GV or CDX-011) in patients with metastatic gpNMB-overexpressing triple-negative breast cancer (TNBC). Cancer Research 2016;76(4 Suppl):Abstract no OT1-03-15. [DOI: ]
Mobarek 2009 {published data only}
-
- Mobarek NA, Hashem TAM. Cisplatin vinorelbine versus cisplatin capecitabine in the treatment of metastatic breast cancer. Annals of Oncology 2009;20:ii63.
Moiseenko 2000a {published data only}
-
- Moiseenko VM, O'Reilly SM, Dalbot DC, Belle S, Gordon RJ, Griffin T, et al. [A comparative randomized phase-II study of Xeloda (capecitabine) and paclitaxel in patients with breast cancer progressing after anthracycline antibiotics]. Voprosy onkologii 2000;46(3):285-9. - PubMed
-
- Molseyenko VO, O'Reilly SM, Talbot D, Gordon RJ, Griffin T, Osterwalder B. A randomized phase II study of XelodaTM (capecitabine) vs paclitaxel In breast cancer patients failing previous anthracycline therapy. Annals of Oncology 1998;9(13):Abstract no 62.
-
- O'Reilly SM, Moiseenko VM, Talbot D, Gordon RJ, Griffin T, Osterwalder B. A randomized phase II study of Xeloda (capecitabine) vs paclitaxel in breast cancer patients failing previous anthracycline therapy. Proceedings of American Society of Clinical Oncology 1998;17:163a.
-
- Talbot DC, Moiseyenko V, Van Belle S, O'Reilly SM, Alba Conejo E, Ackland S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. British Journal of Cancer 2002;86(9):1367-72. [DOI: 10.1038/sj.bjc.6600261] - DOI - PMC - PubMed
Nagayama 2012 {published data only}
-
- Nagayama A, Jinno H, Takahashi M, Hayashida T, Hirose S, Kitagawa Y. Abstract P1-14-09: Immunohistochemical classification of intrinsic subtypes as a predictive biomarker of pathological complete response in breast cancer patients treated with preoperative chemotherapy. Cancer Research 2012;72(24 Suppl):P1-14-09. [DOI: 10.1158/0008-5472.sabcs12-p1-14-09] - DOI
NCT00081796 {published data only}
-
- NCT00081796. Breast Cancer Trial of RPR109881 Versus Capecitabine in Male or Female Patients With Advanced Breast Cancer. clinicaltrials.gov/ct2/show/NCT00081796 (first received 22 April 2004).
NCT00082095 {published data only}
-
- NCT00082095. To compare treatment with doxorubicin or capecitabine for metastatic breast cancer in women 60 years and older. clinicaltrials.gov/ct2/show/NCT00082095 (first received 30 April 2004).
NCT01112826 {published data only}
-
- NCT01112826. Efficacy of capecitabine metronomic chemotherapy to triple negative breast cancer (SYSUCC001). clinicaltrials.gov/ct2/show/NCT01112826 (first received 28 April 2010).
NCT01354522 {published data only}
-
- NCT01354522. TAC versus TCX as adjuvant treatment for node-positive Her2-negative breast cancer. clinicaltrials.gov/ct2/show/NCT01354522 (first received 17 May 2011). [NCT01354522]
NCT01415336 {published data only}
-
- NCT01415336. AX versus AC as adjuvant treatment for node-negative breast cancer. clinicaltrials.gov/ct2/show/NCT01415336 (first received 11 August 2011).
NCT01655992 {published data only}
-
- NCT01655992. A trial comparing S1 generic with capecitabine in metastatic breast cancer (MBC). clinicaltrials.gov/ct2/show/NCT01655992 (first received 2 August 2012).
NCT01869192 {published data only}
-
- NCT01869192. Phase II trial for large ER-negative breast cancers. clinicaltrials.gov/ct2/show/NCT01869192 (first received 5 June 2013).
NCT02207335 {published data only}
-
- NCT02207335. Trial of gemcitabine capecitabine versus gemcitabine carboplatin in breast cancer. clinicaltrials.gov/ct2/show/NCT02207335 (first received 4 August 2014).
NCT02767661 {published data only}
-
- NCT02767661. Metronomic capecitabine plus aromatase inhibitor for first line treatment in HR(+), Her2(-) metastatic breast cancer (MECCA). clinicaltrials.gov/ct2/show/NCT02767661 (first received 10 May 2016).
NorCap‐CA223 {published data only}2005‐000438‐19
-
- Cinieri S, Chan A, Altundag K, Tubiana-Mathieu N, Barnadas A, Dodyk P, et al. Final results of an international three-arm randomised phase II study evaluating oral vinorelbine plus capecitabine versus paclitaxel plus gemcitabine versus docetaxel plus gemcitabine as first-line chemotherapy in patients with metastatic breast cancer (NorCap-CA223 trial). European Journal of Cancer 2013;49:S417.
-
- Cinieri S, Chan A, Altundag K, Vandebroek A, Tubiana-Mathieu N, Barnadas A, et al. Three-arm randomized phase II study evaluating oral vinorelbine plus capecitabine versus paclitaxel plus gemcitabine versus docetaxel plus gemcitabine as first-line chemotherapy in patients with metastatic breast cancer: Final results (NorCap-CA223 trial). Journal of Clinical Oncology 2014;32(15):1044.
O'Shaughnessy 2001 {published data only}
-
- O'Shaughnessy J, Moiseyenko V, Bell D, Nabholtz JM, Miles D, Gorbunova V, et al. A randomized phase II study of Xeloda (TM) (capecitabine) vs CMF as first line chemotherapy of breast cancer in women aged 55 years. Proceedings of the Annual Meeting of the American Society of Oncology 1998:Abstract 398.
-
- O'Shaughnessy JA, Blum J, Moisenyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Annals of Oncology 2001;12(9):1247-54. - PubMed
OMEGA {published data only}2006‐002046‐1011114726
-
- Hamaker M, Seynaeve CM, Wymenga M, Tinteren H, Nortier JWR, Maartense E, et al. Baseline comprehensive geriatric assessment to predict for toxicity of single-agent chemotherapy in elderly metastatic breast cancer patients: Results from the OMEGA study of the Dutch Breast Cancer Trialists' Group. Journal of Clinical Oncology 2012;30(15 Suppl):1080.
-
- Hamaker ME, Seynaeve C, Wymenga AN, Tinteren H, Nortier JW, Maartense E, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch breast cancer trialists' group. Breast 2014;23(1):81-7. - PubMed
-
- Seynaeve C, Tinteren H, Wymenga ANM, Nortier JWR, Maartense E, Jongh FE, et al. 249 Feasibility and toxicities associated with PEG doxo versus capecitabine as first-linechemotherapy in elderly metastatic breast cancer (MBC) patients; results from the randomized OMEGA study of the Dutch Breast Cancer Trialists’ Group (BOOG). European Journal of Cancer 2012;48(S115):Abstract 249. [DOI: 10.1016/S0959-8049(12)70316-7] - DOI
-
- Smorenburg CH, De Groot SM, Van Leeuwen-Stok AE, Hamaker ME, Wymenga AN, De Graaf H, et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: Results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Annals of Oncology 2014;25(3):599-605. [DOI: 10.1093/annonc/mdt588] - DOI - PMC - PubMed
-
- Smorenburg CH, Seynaeve C, Wymenga MANM, Maartense E, Graaf H, Jongh FE, et al. First-line chemotherapy with pegylated liposomal doxorubicin versus capecitabine in elderly patients with metastatic breast cancer: Results of the phase iii omega study of the Dutch Breast Cancer Trialists' Group (BOOG). Cancer Research 2012;72(24 Suppl):Abstract no P1-12-05. [DOI: 10.1158/0008-5472.sabcs12-p1-12-05] - DOI
OOTR N003 {published data only}C000000322
-
- Ohno S, Chow LW, Sato N, Masuda N, Sasano H, Takahashi F, et al. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil- epirubicin-cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Research & Treatment 2013;142(1):69-80. [DOI: 10.1007/s10549-013-2691-y] - DOI - PMC - PubMed
-
- Toi M, Ohno S, Sato N, Masuda N, Sasano H, Takahashi F, et al. Abstract P1-14-02: Preoperative docetaxel (T) with or without capecitabine (X) following epirubicin, 5-fluorouracil and cyclophosphamide (FEC) in patients with operable breast cancer (OOTR N003): Results of comparative study and predictive marker analysis. Cancer Research 2012;72(24 Suppl):Abstract P1-14-02. [DOI: 10.1158/0008-5472.sabcs12-p1-14-02] - DOI
-
- Toi M, Takada M, Sugimoto M, Naito Y, Bando H, Iwata H, et al. Development of a prediction model for treatment response to neoadjuvant chemotherapy in patients with primary breast cancer using a decision-tree algorithm. Annals of Surgical Oncology 2012;19:S69. - PubMed
Pegram 2005 {published data only}
-
- Pegram M. Phase III randomized study of XRP9881 versus capecitabine in patients with locally recurrent inoperable or metastatic breast cancer that progressed after prior taxane- and anthracycline-based therapy. journals.lww.com/oncology-times/fulltext/2005/05100/protocol_alert.13.aspx (accessed 2019).
PELICAN {published data only}2005‐003164‐35‐DE
-
- Al-Batran S, Saupe S, Kerber A, Lueck HJ, Jackisch C, Untch M, et al. Interim safety analysis of a randomized phase III study evaluating pegylated liposomal doxorubicin (PLD) versus capecitabine as first line chemotherapy for metastatic breast cancer (MBC) – The PELICAN study. European Journal of Cancer Supplements 2008;6(7):177. [DOI: 10.1016/S1359-6349(08)70740-7] - DOI
-
- Al-Batran S, Saupe S, Schmidt M, Kreienberg R, Otremba B, Warm M, et al. Interim safety analysis of the randomized phase III PELICAN trial evaluating pegylated liposomal doxorubicin (PLD) versus capecitabine as first-line therapy for metastatic breast cancer. Journal of Clinical Oncology 2009;27(15):1118.
-
- De Wit M, Harbeck N, Scholz M, Lerbs W, Wedding U, Honecker F. Incorporation of a Comprehensive Geriatric Assessment (CGA) into a randomized phase III trial for metastatic breast cancer: The PELICAN Study. Journal of Clinical Oncology 2009;27(15):9551.
-
- De Wit M, Honecker F, Wedding U, Waldenmaier D, Dorn J, Warm M, et al. Abstract P6-11-06: Correlation Comprehensive Geriatric Assessment (CGA) and inflammation and nutrition parameters with outcome measures in the phase III PELICAN Trial for Metastatic Breast Cancer (MBC) 929 2625. Cancer Research 2010;70(24 Suppl):6-11.
-
- De Wit M, Honecker F, Wedding U, Waldenmaier D, Dorn J, Warm M, et al. Incorporation of a comprehensive geriatric assessment (CGA) into a randomized phase III trial for metastatic breast cancer (MBC): The PELICAN study. Journal of Clinical Oncology 2010;28(15 Suppl):1070.
Pivot 2016 {published data only}
RIBBON‐1 {published data only}
-
- Bondarenko I, Glaspy J, Brufsky A, Lipatov O, Perez EA, Chan S, et al. 476 PFS by patient subgroup for standard chemotherapies in combination with bevacizumab (BV) in the first-line treatment of HER2-negative locally recurrent (LR) or metastatic breast cancer (mBC): results from RIBBON-1. European Journal of Cancer Supplements 2010;8(3):198. [DOI: ]
-
- Bondarenko I, Glaspy J, Brufsky A, Lipatov O, Perez EA, Chan S, et al. PFS by patient subgroup for standard chemotherapies in combination with bevacizumab (BV) in the first-line treatment of HER2-negative locally recurrent (LR) or metastatic breast cancer (mBC): Results from RIBBON-1. European Journal of Cancer Supplement 2010;8(3):198.
-
- Brufsky A, Ponomarova O, Tjulandin S. 485 Influence of disease free interval on the efficacy of capecitabine-bevacizumab for HER2-negative metastatic breast cancer (MBC) in the RIBBON-1 trial. European Journal of Cancer Supplements 2010;8(3):201. [DOI: ]
-
- Brufsky A. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer: Robert NJ, Dieras V, Glaspy J, et al (Virginia Cancer Specialists, Fairfax; Univ of California, Los Angeles; Genentech, South San Francisco, CA; et al). Breast Diseases 2011;22(4):412-3. [DOI: 10.1016/j.breastdis.2011.10.016] - DOI - PubMed
-
- Dieras V, Semiglazov V, Tjulandin S. Efficacy of first-line capecitabine plus bevacizumab in patients with ER/PgR-positive metastatic breast cancer (MBC) and those previously treated with hormone therapy. European Journal of Cancer Supplements 2010;8(3):202.
RIBBON‐2 {published data only}
-
- Brufsky A, Bondarenko I, Smirnov V, Hurvitz S, Perez E, Ponomarova O, et al. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of HER2-negative metastatic breast cancer. Cancer Research 2009;69(24 Suppl):Abstract no 42. - PubMed
-
- Brufsky A, Bondarenko IN, Smirnov V. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of HER2-negative metastatic breast cancer. Clinical Advances in Hematology and Oncology 2010;8(1):17. - PubMed
-
- Brufsky A, Rivera RR, Hurvitz SA, Bondarenko IN, Smirnov V, Valero V, et al. Progression-free survival (PFS) in patient subgroups in RIBBON-2, a phase III trial of chemotherapy (chemo) plus or minus bevacizumab (BV) for second-line treatment of HER2-negative, locally recurrent or metastatic breast cancer (MBC). Journal of Clinical Oncology 2010;28(15 Suppl):1021.
-
- Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: Subgroup analysis of the RIBBON-2 trial. Breast Cancer Research and Treatment 2012;133(3):1067-75. [DOI: 10.1007/s10549-012-2008-6] - DOI - PubMed
-
- Brufsky A, Valero V, Tiangco B, Dakhil SR, Brize A, Bousfoul N, et al. Impact of bevacizumab (BEV) on efficacy of second-line chemotherapy (CT) for triple-negative breast cancer (TNBC): analysis of RIBBON-2. Journal of Clinical Oncology 2011;29(15 Suppl):1010.
Rivera 2012 {published data only}
-
- Rivera E, Chang JC, Semiglazov V, Gorbunova V, Manikhas A, Krasnozhon D, et al. Abstract OT3-3-01: Eniluracil + 5-fluorouracil + leucovorin (EFL) vs. capecitabine phase 2 trial for metastatic breast cancer. Cancer Research 2012;72(24):Abstract OT3-3-01. [DOI: 10.1158/0008-5472.sabcs12-ot3-3-01] - DOI
Rivera Rodriguez 2013 {published data only}
-
- Rivera-Rodriguez N, Cabanillas F, Lawrenson L, Negron V, Pavia O, Bruno M, et al. Abstract P3-14-17: Results of a novel neoadjuvant chemotherapy (NAC) for breast cancer. Cancer Research 2013;73(24 Suppl):Abstract no P3-14-17. [DOI: 10.1158/0008-5472.sabcs13-p3-14-17] - DOI
Roche 2006 {published data only}
-
- Roche H, Deporte R, Berton RD, Coudert B, Tubiana MN, Ferrero J, et al. Capecitabine + epirubicin + cyclophosphamide (CEX) has comparable safety to 5-FU + epirubicin + cyclophosphamide (FEC) as neoadjuvant therapy in patients (pts) with operable breast cancer (BC): Early safety findings from a randomized phase III trial. Journal of Clinical Oncology 2006;24(18 Suppl):10655.
Rugo 2008 {published data only}
-
- Rugo H. Combined therapy: Ixempra plus xeloda. P and T 2008;33(3):149.
SAKK 24/09 {published data only}
-
- Rochlitz C, Von Moos R, Bigler M, Zaman K, Anchisi S, Kung M, et al. SAKK 24/09: Safety and tolerability of bevacizumab plus paclitaxel versus bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer-A multicenter, randomized phase III trial. Journal of Clinical Oncology 2014;32(15 Suppl):518. - PMC - PubMed
Sato 2012 {published data only}
-
- Sato N, Yamamoto D, Rai Y, Iwase H, Saito M, Iwata H, et al. Abstract P1-12-01: Evaluation on efficacy and safety of capecitabine plus docetaxel versus docetaxel monotherapy in metastatic breast cancer patients pretreated with anthracycline: Results from a randomized phase III study (JO21095). Cancer Research 2012;72(24 Suppl):Abstract no P1-12-01. [DOI: 10.1158/0008-5472.sabcs12-p1-12-01] - DOI
Schneeweiss 2013 {published data only}
-
- Schneeweiss A, Fett W, Aktas B, Fruehauf S, Grafe A, Jakob A, et al. Abstract P4-14-04: AVANTI: A non-interventional study examining the combination of bevacizumab with paclitaxel or capecitabine in metastatic breast cancer. Cancer Research 2013;73(24 Suppl):P4-14-04. [DOI: 10.1158/0008-5472.sabcs13-p4-14-04] - DOI
Shao 2010 {published data only}
-
- Shao Z, Huang B, Zhang J, Zhou S, He P, Chen D, et al. First interim analysis of a randomized trial comparing capecitabine/epirubicin/cyclophosphamide (XEC) vs 5-FU/epirubicin/ cyclophosphamide (FEC) as adjuvant therapy for medium- or high-risk early breast cancer (EBC). European Journal of Cancer Supplements 2010;8(3):64.
Soto 2006 {published data only}
-
- Soto C, Torrecillas L, Reyes S, Ramirez M, Perez L, Cervantes G, et al. Capecitabine (X) and taxanes in patients (pts) with anthracycline-pretreated metastatic breast cancer (MBC): Sequential vs. combined therapy results from a MOSG randomized phase III trial. Journal of Clinical Oncology 2006;24(18 Suppl):570.
TANIA {published data only}
-
- Cortes J, Vrdoljak E, Puglisi F, Marschner N, Gligorov J, Zielinski C, et al. Abstract P3-06-08: Plasma (p) biomarker results from the TANIA trial evaluating continued or reintroduced bevacizumab (BEV) after 1st-line BEV for HER2-negative metastatic breast cancer (mBC). Cancer Research 2015;75(9 Suppl):P3-06-08. [DOI: 10.1158/1538-7445.sabcs14-p3-06-08] - DOI
-
- Puglisi F, Cortes J, Vrdoljak E, Gligorov J, Marschner N, Zielinski C, et al. Abstract PD2-4: Subgroup efficacy analyses of the randomized phase III TANIA trial evaluating continued or reintroduced bevacizumab (BEV) after 1st-line BEV for HER2-negative locally recurrent/metastatic breast cancer (LR/mBC). Cancer Research 2015;75(9 Suppl):PD2-4. [DOI: 10.1158/1538-7445.sabcs14-pd2-4] - DOI
-
- Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): An open-label, randomised phase 3 trial. The Lancet Oncology 2014;15(11):1269-78. [DOI: ] - PubMed
-
- Vrdoljak E, Marschner N, Zielinski C, Gligorov J, Cortes J, Puglisi F, et al. Final results of the TANIA randomised phase III trial of bevacizumab after progression on first-line bevacizumab therapy for HER2-negative locally recurrent/metastatic breast cancer. Annals of Oncology 2016;27(11):2046-52. - PubMed
TEX {published data only}
-
- Hatschek T, Carlsson L, Einbeigi Z, Lidbrink E, Linderholm B, Lindh B, et al. Individually tailored treatment with epirubicin and paclitaxel with or without capecitabine as first-line chemotherapy in metastatic breast cancer: a randomized multicenter trial. Breast Cancer Research and Treatment 2012;131(3):939-47. [DOI: 10.1007/s10549-011-1880-9] - DOI - PubMed
-
- Hatschek T, Einbeigi Z, Walz T, Malmberg M, Loman N, Carlsson L, et al. Individually dose-adjusted treatment with epirubicin and paclitaxel with or without capecitabine as 1st line treatment in metastatic breast cancer. A randomized multicenter trial. European Journal of Cancer Supplements 2010;8(3):195-6.
-
- Hedenfalk I, Kimbung S, Kovacs A, Skoog L, Einbeigi Z, Walz T, et al. Prognostic relevance of Claudin-2 expression in metastatic breast cancer. Cancer Research 2012;72(24 Suppl):P1-05-07. [DOI: 10.1158/0008-5472.sabcs12-p1-05-07] - DOI
-
- Suzuki C, Blomqvist L, Hatschek T, Carlsson L, Einbeigi Z, Linderholm B, et al. Impact of the first tumor response at eight weeks on overall survival in metastatic breast cancer patients treated with first-line combination chemotherapy. Medical Oncology 2013;30(1):Article no 415. [DOI: 10.1007/s12032-012-0415-5] - DOI - PubMed
-
- Svensson H, Einbeigi Z, Johansson H, Hatschek T, Brandberg Y. Abstract P3-10-29: Health-related quality of fife (HRQoL) as prognostic factor for time to progression (TTP) and survival in women with metastatic breast cancer. Cancer Research 2010;70(24 Suppl):P3-10-29.
VITAL {published data only}2009‐009885‐15
-
- Janni W, Alvarez JV, Papadimitriou CA, Karaszewska B, Wiest W, Lim ML, et al. A phase II randomized trial of lapatinib with either vinorelbine or capecitabine in ErbB2-overexpressing first- and second-line metastatic breast cancer (MBC). Journal of Clinical Oncology 2010;28(15 Suppl):TPS135.
-
- Janni W, Pikiel J, Sarosiek T, Karaszewska B, Papadimitriou CA, Schwedler K, et al. A phase II randomized trial of lapatinib with either vinorelbine or capecitabine as first- and second-line therapy for HER2-overexpressing metastatic breast cancer. Cancer Research 2011;71(24 Suppl):OT1-02-09.
-
- Janni W, Sarosiek T, Karaszewska B, Pikiel J, Staroslawska E, Potemski P, et al. A phase II, randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer. Breast Cancer Research and Treatment 2014;143(3):493-505. [DOI: ] - PMC - PubMed
-
- Janni W, Sarosiek T, Karaszewska B, Pikiel J, Staroslawska E, Potemski P, et al. Final overall survival analysis of a phase II trial evaluating vinorelbine and lapatinib in women with ErbB2 overexpressing metastatic breast cancer. Breast 2015;24(6):769-73. - PubMed
-
- Janni W, Sarosiek T, Papadimitriou CA, Alvarez Gallego JV, Caruso M, Wiest W, et al. A phase II randomized trial of lapatinib with either vinorelbine or capecitabine as first- and second-line therapy for ErbB2-overexpressing metastatic breast cancer (MBC): Safety results. Journal of Clinical Oncology 2011;29(15 Suppl):e11097.
Wang 2014 {published data only}
-
- Wang T, Li W, Zhang HS, Bian L, Song TS, Jiang Z. 397P - Study comparing trastuzumab plus vinorelbine with trastuzumab plus capecitabine in heavily pretreated Her-2 positive metastatic breast cancer. Annals of Oncology 2014;25(Suppl 4):iv131. [DOI: 10.1093/annonc/mdu329.46] - DOI
Wang 2015 {published data only}
-
- Wang J, Xu B, Yuan P, Ma F, Li Q, Zhang P, et al. Capecitabine combined with docetaxel versus vinorelbine followed by capecitabine maintenance medication for first-line treatment of patients with advanced breast cancer: Phase 3 randomized trial. Cancer 2015;121(19):3412-21. [DOI: ] - PubMed
XeNA {published data only}
Yamamoto 2014 {published data only}
-
- Yamamoto D, Iwase S, Yamaguchi T, Tsubota Y, Kawaguchi T, Odagiri H, et al. Patient preference trial comparing capecitabine and S-1 in metastatic breast cancer patients. Journal of Clinical Oncology 2014;32(15 Suppl):TPS2634.
Yang 2013 {published data only}
-
- Yang B, Yang JL, Shi WW, Liu H, Zhu YY, Fang P, et al. [Clinical paired study of comparing docetaxel plus capecitabine versus docetaxel plus epirubicin as first-line treatment in women with HER-2 negative advanced breast cancer]. Zhonghua Yi Xue Za Zhi 2013;93(18):1397-400. - PubMed
Yardley 2015 {published data only}
-
- Yardley DA, Melisko ME, Forero A, Daniel BR, Montero AJ, Guthrie TH, et al. METRIC: A randomized international study of the antibody-drug conjugate glembatumumab vedotin (GV or CDX-011) in patients (pts) with metastatic gpNMBo-verexpressing triple-negative breast cancer (TNBC). Journal of Clinical Oncology 2015;33(15 Suppl):TPS1110.
Yoshinami 2013 {published data only}
-
- Yoshinami T, Nakayama T, Ikeda M, Iwamoto M, Komoike Y, Takashima T, et al. Abstract OT3-1-01: A randomized phase II study of maintenance hormone therapy with or without capecitabine after induction chemotherapy with bevacizumab plus paclitaxel in hormone receptor positive and HER2 negative metastatic breast cancer (KBCSG-TR1214). Cancer Research 2013;73(24 Suppl):OT3-1-01. [DOI: 10.1158/0008-5472.sabcs13-ot3-1-01] - DOI
Yu 2011 {published data only}
-
- Yu J, Lj DI, Song Gh, Che L, Jiang HF, Zhu YL, et al. [Randomized clinical case-control trial for the comparison of docetaxel plus thiotepa versus docetaxel plus capecitabine in patients with metastatic breast cancer]. [Chinese]. Beijing da Xue Xue Bao 2011;Yi(1):151-6. - PubMed
Zhang 2015 {published data only}
-
- Zhang X, Zhou Y, Mao F, Lin Y, Guan J, Sun Q. Efficacy and safety of pirarubicin plus capecitabine versus pirarubicin plus cyclophosphamide in Chinese node-negative breast cancer patients: a 4-year open-label, randomized, controlled study. Medical Oncology 2015;32(10):240. - PubMed
Additional references
Andre 2010
Blum 2012
-
- Blum J, Barrios C, Feldman N, Verma S, McKenna E, Lee L, et al. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Research and Treatment 2012;136(3):777-88. - PubMed
Braun 2000
-
- Braun S, Kentenich C, Janni W, Hepp F, Waal J, Willgeroth F, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. Journal of Clinical Oncology 2000;18(1):80–6. - PubMed
Bray 2018
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394-424. - PubMed
EBCTCG 2005
-
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365(9472):1687-717. - PubMed
Eisenhauer 2009
-
- Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228-47. - PubMed
FDA 2014
-
- FDA 2014. Roche Xeloda (capecitabine) tablets. FDA Access Data (accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf) (accessed 17 January 2014).
Ferguson 2007
Ferlay 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0. IARC Cancer Base No. 11 2012.
Gerratana 2016
-
- Gerratana L, Fanotto V, Pelizzari G, Agostinetto E, Puglisi F. Do platinum salts fit all triple negative breast cancers? Cancer Treatment Reviews 2016;48:34-41. - PubMed
Gluck 2009
-
- Gluck S, Russel C, O'Shaughnessy J, Yuan G, Odom P, Sherrill B, et al. Relationship between survival and estrogen receptor (ER) status in patients with metastatic breast cancer (MBC) treated with capecitabine (C) and docetaxel (D): an exploratory data analysis. Journal of Clinical Oncology 2009;27(15 Suppl):1024.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Horiguchi 2004
-
- Horiguchi J, Yoshida T, Koibuchi Y, Iijima K, Ninomiya J, Takei H, et al. DPD activity and immunohistochemical DPD expression in human breast cancer. Oncology Reports 2004;11(1):65–72. - PubMed
Houssami 2012
-
- Houssami N, Macaskill P, Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy.. European Journal of Cancer 2012;48(18):3342-54. - PubMed
Kim 2012
Lee 2008
-
- Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Research and Treatment 2008;109(3):481-9. - PubMed
Lee 2011
-
- Lee SJ, Choi YL, Park YH, Kim ST, Cho EY, Ahn JS, et al. Thymidylate synthase and thymidine phosphorylase as predictive markers of capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemotherapy and Pharmacology 2011;68(3):743–51. - PubMed
Li 2013
Mackelenbergh 2019
-
- Mackelenbergh M, Seither F, Möbus V, O'Shaugnessy J, Martin M, Joenssuu H, et al. Abstract GS1-07: Effects of capecitabine as part of neo-/adjuvant chemotherapy. A meta-analysis of individual patient data from 12 randomized trials including 15,457 patients. Cancer Research 2020;80(4 Suppl):GS1-07. [DOI: 10.1158/1538-7445.SABCS19-GS1-07] - DOI
Martin 2015
-
- Martin HL, Ohara K, Chin W, Davidson A, Bayliss E, Redfern A, Khattak MA. Cancer services in Western Australia: a comparison of regional outcomes with metropolitan Perth. Australian Journal of Rural Health 2015;23(5):302-8. - PubMed
Merino 2016
-
- Merino D, Lok SW, Visvader JE, Lindeman GJ. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016;35(15):1877-87. - PubMed
Miwa 1998
-
- Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. European Journal of Cancer 1998;34(8):1274-81. - PubMed
Moja 2012
Natori 2017
-
- Natori A, Ethier JL, Amir E, Cescon DW. Capecitabine in early breast cancer: a meta-analysis of randomised controlled trials. European Journal of Cancer 2017;77:40-7. - PubMed
Osako 2009
-
- Osako T, Ito Y, Ushijima M, Takahashi S, Tokudome N, Sugihara T, et al. Predictive factors for efficacy of capecitabine in heavily pretreated patients with metastatic breast cancer. Cancer Chemotherapy and Pharmacology 2009;63(5):865-71. - PubMed
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. 1998 Statistics in Medicine;17(24):2815-34. - PubMed
Ray 2013
-
- Ray S, Bonthapally V, McMorrow D, Bonafede M, Landsman-Blumberg P. Patterns of treatment, healthcare utilization and costs by lines of therapy in metastatic breast cancer in a large insured US population. Journal of Comparative Effectiveness Research 2013;2(2):195-206. - PubMed
RevMan [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Seidman 2014
Siva 2008
-
- Siva P, Correa P, Skaria S, Canney P. Capecitabine in advanced breast cancer: predictive factors for response. Journal of Clinical Oncology 2008;26(15 Supplement):1126.
Tierney 2007
von Minckwitz 2008
-
- Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio Trial. Journal of the National Cancer Institute 2008;100(8):542-51. - PubMed
Wang 2012
-
- Wang Y, Yang H, Wei JF, Meng L. Efficacy and toxicity of capecitabine-based chemotherapy in patients with metastatic or advanced breast cancer: results from ten randomized trials. Current Medical Research and Opinion 2012;28(12):1911-9. - PubMed
Yoshikawa 2001
-
- Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, et al. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Research 2001;61(3):1029–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

