Randomised phase 3 open-label trial of first-line treatment with gemcitabine in association with docetaxel or paclitaxel in women with metastatic breast cancer: a comparison of different schedules and treatments
- PMID: 23537313
- PMCID: PMC3621657
- DOI: 10.1186/1471-2407-13-164
Randomised phase 3 open-label trial of first-line treatment with gemcitabine in association with docetaxel or paclitaxel in women with metastatic breast cancer: a comparison of different schedules and treatments
Abstract
Background: This open-label study compared docetaxel/gemcitabine vs. paclitaxel/gemcitabine and a weekly (W) vs. 3-weekly (3 W) schedule in metastatic breast cancer (MBC).
Methods: Patients relapsed after adjuvant/neoadjuvant anthracycline-containing chemotherapy were randomized to: A) gemcitabine 1000 mg/m2 Day 1,8 + docetaxel 75 mg/m2 Day 1 q3W; B) gemcitabine 1250 mg/m2 Day 1,8 + paclitaxel 175 mg/m2 Day 1 q3W; C) gemcitabine 800 mg/m2 Day 1,8,15 + docetaxel 30 mg/m2 Day 1,8,15 q4W; D) gemcitabine 800 mg/m2 Day 1,15 + paclitaxel 80 mg/m2 Day 1,8,15 q4W. Primary endpoint was time-to-progression (TTP). Secondary endpoints were overall survival (OS) and overall response rate (ORR).
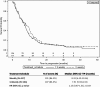
Results: Interim analysis led to accrual interruption (241 patients enrolled of 360 planned). Median TTP (months) was 8.33 (95% CI: 6.19-10.16) with W and 7.51 (95% CI: 5.93-8.33) with 3 W (p=0.319). No differences were observed in median TTP between docetaxel and paclitaxel, with 85.6% and 87.0% of patients progressing, respectively. OS did not differ between regimens/schedules. ORR was comparable between regimens (HR: 0.882; 95% CI: 0.523-1.488; p=0.639), while it was significantly higher in W than in the 3 W (HR: 0.504; 95% CI: 0.299-0.850; p=0.010) schedule. Grade 3/4 toxicities occurred in 69.2% and 71.9% of patients on docetaxel and paclitaxel, and in 65.8% and 75.2% in W and 3 W.
Conclusions: Both treatment regimens showed similar TTP. W might be associated with a better tumour response compared with 3 W.
Trial registration: Clinicaltrial.gov ID NCT00236899.
Figures



References
-
- Cardoso F, Fallowfield L, Costa A. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi25–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

