The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: an open-label pilot study
- PMID: 18005455
- PMCID: PMC2244616
- DOI: 10.1186/1471-2431-7-36
The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: an open-label pilot study
Abstract
Background: Recently, hyperbaric oxygen therapy (HBOT) has increased in popularity as a treatment for autism. Numerous studies document oxidative stress and inflammation in individuals with autism; both of these conditions have demonstrated improvement with HBOT, along with enhancement of neurological function and cognitive performance. In this study, children with autism were treated with HBOT at atmospheric pressures and oxygen concentrations in current use for this condition. Changes in markers of oxidative stress and inflammation were measured. The children were evaluated to determine clinical effects and safety.
Methods: Eighteen children with autism, ages 3-16 years, underwent 40 hyperbaric sessions of 45 minutes duration each at either 1.5 atmospheres (atm) and 100% oxygen, or at 1.3 atm and 24% oxygen. Measurements of C-reactive protein (CRP) and markers of oxidative stress, including plasma oxidized glutathione (GSSG), were assessed by fasting blood draws collected before and after the 40 treatments. Changes in clinical symptoms, as rated by parents, were also assessed. The children were closely monitored for potential adverse effects.
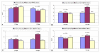
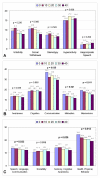
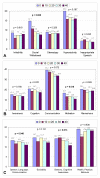
Results: At the endpoint of 40 hyperbaric sessions, neither group demonstrated statistically significant changes in mean plasma GSSG levels, indicating intracellular oxidative stress appears unaffected by either regimen. A trend towards improvement in mean CRP was present in both groups; the largest improvements were observed in children with initially higher elevations in CRP. When all 18 children were pooled, a significant improvement in CRP was found (p = 0.021). Pre- and post-parental observations indicated statistically significant improvements in both groups, including motivation, speech, and cognitive awareness (p < 0.05). No major adverse events were observed.
Conclusion: In this prospective pilot study of children with autism, HBOT at a maximum pressure of 1.5 atm with up to 100% oxygen was safe and well tolerated. HBOT did not appreciably worsen oxidative stress and significantly decreased inflammation as measured by CRP levels. Parental observations support anecdotal accounts of improvement in several domains of autism. However, since this was an open-label study, definitive statements regarding the efficacy of HBOT for the treatment of individuals with autism must await results from double-blind, controlled trials.
Trial registration: clinicaltrials.gov NCT00324909.
Figures

References
-
- CDC Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, six cities, United States. 2000. MMWR. 2007;56:1–40. - PubMed
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994.
-
- Charman T, Taylor E, Drew A, Cockerill H, Brown JA, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. J Child Psychol Psychiatry. 2005;46:500–513. doi: 10.1111/j.1469-7610.2004.00377.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

