Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500
- PMID: 24888818
- PMCID: PMC4209100
- DOI: 10.1200/JCO.2014.56.2561
Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500
Abstract
Purpose: Increased circulating tumor cells (CTCs; five or more CTCs per 7.5 mL of whole blood) are associated with poor prognosis in metastatic breast cancer (MBC). A randomized trial of patients with persistent increase in CTCs tested whether changing chemotherapy after one cycle of first-line chemotherapy would improve the primary outcome of overall survival (OS).
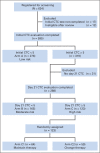
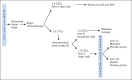
Patients and methods: Patients with MBC who did not have increased CTCs at baseline remained on initial therapy until progression (arm A). Patients with initially increased CTCs that decreased after 21 days of therapy remained on initial therapy (arm B). Patients with persistently increased CTCs after 21 days of therapy were randomly assigned to continue initial therapy (arm C1) or change to an alternative chemotherapy (arm C2).
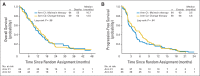
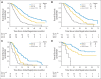
Results: Of 595 eligible and evaluable patients, 276 (46%) did not have increased CTCs (arm A). Of those with initially increased CTCs, 31 (10%) were not retested, 165 were assigned to arm B, and 123 were randomly assigned to arm C1 or C2. No difference in median OS was observed between arm C1 and C2 (10.7 and 12.5 months, respectively; P = .98). CTCs were strongly prognostic. Median OS for arms A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P < .001).
Conclusion: This study confirms the prognostic significance of CTCs in patients with MBC receiving first-line chemotherapy. For patients with persistently increased CTCs after 21 days of first-line chemotherapy, early switching to an alternate cytotoxic therapy was not effective in prolonging OS. For this population, there is a need for more effective treatment than standard chemotherapy.
Trial registration: ClinicalTrials.gov NCT00382018.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Solidifying liquid biopsies: can circulating tumor cell monitoring guide treatment selection in breast cancer?J Clin Oncol. 2014 Nov 1;32(31):3470-1. doi: 10.1200/JCO.2014.57.1505. Epub 2014 Jul 14. J Clin Oncol. 2014. PMID: 25024075 No abstract available.
-
Reply to F.-C. Bidard et al.J Clin Oncol. 2015 May 10;33(14):1623. doi: 10.1200/JCO.2014.60.5519. Epub 2015 Apr 13. J Clin Oncol. 2015. PMID: 25870090 No abstract available.
-
Clinical utility of circulating tumor cells in metastatic breast cancer.J Clin Oncol. 2015 May 10;33(14):1622. doi: 10.1200/JCO.2014.57.9714. Epub 2015 Apr 13. J Clin Oncol. 2015. PMID: 25870091 No abstract available.
References
-
- Clark GM, Sledge GW, Jr, Osborne CK, et al. Survival from first recurrence: Relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5:55–61. - PubMed
-
- Chia SK, Speers CH, D'yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. - PubMed
-
- Lin NU, Thomssen C, Cardoso F, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: Surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast. 2013;22:203–210. - PubMed
-
- Miles D, von Minckwitz G, Seidman AD. Combination versus sequential single-agent therapy in metastatic breast cancer. Oncologist. 2002;6:13–19. - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA105409/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- U10 CA086780/CA/NCI NIH HHS/United States
- U10 CA095860/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- U10 CA074647/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA073590/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

