Quantitative assessment of altered rectal mucosal permeability due to rectally applied nonoxynol-9, biopsy, and simulated intercourse
- PMID: 23325915
- PMCID: PMC3693591
- DOI: 10.1093/infdis/jit030
Quantitative assessment of altered rectal mucosal permeability due to rectally applied nonoxynol-9, biopsy, and simulated intercourse
Abstract
Background: Microbicide toxicity may reduce the efficacy of topical preexposure prophylaxis for human immunodeficiency virus (HIV) transmission. Noninvasive quantitative measures of microbicide toxicity would usefully inform microbicide development.
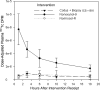
Methods: Ten subjects received 3 one-time interventions: 5 mL of Normosol-R fluid alone (negative control), 5 mL of 2% nonoxynol-9 (N-9) gel, and 5 mL of Normosol-R with coital simulation and sigmoidoscopic biopsy (CS + BX). Each dose of N-9 and Normosol-R contained 500 µCi of (99m)technetium-diethylene triamine pentaacetic acid. Plasma and urine radioactivity was assessed over 24 hours.
Results: The plasma radioisotope concentration peaked 1 hour after N-9 dosing. The mean maximum radioisotope concentration after N-9 receipt was 12.0 times (95% confidence interval [CI], 6.8-21.0) and 8.4 times (95% CI, 5.2-13.5) the mean concentration after Normosol-R control receipt and CS + BX receipt, respectively; paired differences persisted for 24 hours. After N-9 dosing, the urine isotope level was 3.6 times (95% CI, 1.1-11.4) the level observed 8 hours after Normosol-R control receipt and 4.0 times (95% CI, 1.4-11.4) the level observed 4 hours after CS + BX receipt. Permeability after CS + BX receipt was greater than that after Normosol-R control receipt in 0-2-hour urine specimens only (mean permeability, 2.4; 95% CI, 1.0-5.8) but was not greater in blood.
Conclusions: Plasma sampling after rectal radioisotope administration provided quantitative estimates of altered mucosal permeability after chemical and mechanical stresses. Permeability testing may provide a useful noninvasive adjunct to assess the mucosal effects of candidate microbicides. Clinical Trials Registration. NCT00389311.
Figures

References
-
- Centers for Disease Control and Prevention. Subpopulation estimates from the HIV incidence surveillance system—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:985–9. - PubMed
-
- Mosher WD, Chandra A, Jones J. Hyattsville, MD: National Center for Health Statistics; 2005. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Report no. 362. - PubMed
-
- Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. - PubMed
-
- Watts DH, Rabe L, Krohn MA, Aura J, Hillier SL. The effects of three nonoxynol-9 preparations on vaginal flora and epithelium. J Infect Dis. 1999;180:426–37. - PubMed
-
- Rustomjee R, Abdool Karim Q, Abdool Karim SS, Laga M, Stein Z. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS. 1999;13:1511–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

