Phase I study of aflibercept in combination with docetaxel in Japanese patients with advanced solid malignancies
- PMID: 35771301
- PMCID: PMC9395466
- DOI: 10.1007/s10637-022-01267-x
Phase I study of aflibercept in combination with docetaxel in Japanese patients with advanced solid malignancies
Abstract
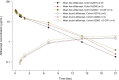
Angiogenesis is a hallmark of cancer development. This study sought to determine the recommended dose of aflibercept, a recombinant fusion protein targeting VEGF-A, VEGF-B and placental growth factor (PlGF), combined with docetaxel in Japanese patients with advanced solid malignancies. This phase I study was planned to include 12 patients following a 3 + 3 algorithm to determine the maximum tolerated dose of aflibercept combined with docetaxel in patients with metastatic or unresectable solid tumors (trial registration: NCT00545246). Docetaxel (75 mg/m2 every 3 weeks or 60 mg/m2 after protocol amendment) was combined with escalating doses of aflibercept (2, 4 and 6 mg/kg every 4 weeks). Free and VEGF-bound aflibercept were measured to assess free aflibercept in excess of the VEGF-bound form. At the starting dose of the combination, 3 of 6 patients treated experienced febrile neutropenia. After reducing the docetaxel dose to 60 mg/m2 in step 2 and permitting therapeutic granulocyte colony-stimulating factor (G-CSF) use, 2 of 3 patients in both cohorts experienced febrile neutropenia. Five patients (42%) had a partial response and 4 patients had stable disease (33%). Free aflibercept in excess of the VEGF-bound form was not maintained at this dose level. The dose limiting toxicity (DLT) of aflibercept combined with docetaxel was febrile neutropenia, which occurred in 2 of 3 Japanese patients at the lowest aflibercept dose level (2 mg/kg) combined with docetaxel (60 mg/m2) and therapeutic G-CSF use. A recommended dose for further studies was not determined because of the DLT at the starting dose.
Keywords: Aflibercept; Docetaxel; Dose escalation; Japanese; VEGF Trap.
© 2022. The Author(s).
Conflict of interest statement
The funding source (Sanofi) was involved in data collection, data analysis, data interpretation, and drafting the manuscript. Yu Sunakawa reports research grants (in the past 36 months) from Chugai Pharma, Taiho Pharma, Takeda, and Otsuka Pharma; and honoraria/lecture fees/speakers’ fees (in the past 36 months) from Eli Lilly Japan, Bristol-Myers Squibb, Chugai Pharma, Ono Pharma, Daiichi-Sankyo, Sysmex, Taiho Pharma, Merck Biopharma, Takeda, Bayer, and Guardant Japan. Keishiro Takahashi and Osamu Kawaguchi are employees of Sanofi and may hold shares and/or stock options in the company. Nobuyuki Yamamoto reports honoraria/lecture fees/speakers’ fees (in the past 36 months) from MSD, Chugai Pharma, Ono Pharma, AstraZeneca, Takeda Pharma, Eli Lilly Japan, Novartis, Pfizer, Janssen, Taiho Pharma, Daiichi-Sankyo, Amgen, Eisai, Toppan Printing, and Boehringer Ingelheim.
Figures
References
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., 3rd Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. - DOI - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical