Adoptive lymphocyte transfer to an HIV-infected progressor from an elite controller
- PMID: 31415245
- PMCID: PMC6795294
- DOI: 10.1172/jci.insight.130664
Adoptive lymphocyte transfer to an HIV-infected progressor from an elite controller
Abstract
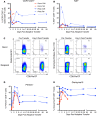
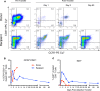
BACKGROUNDHIV-infected patients with poor virologic control and multidrug-resistant virus have limited therapeutic options. The current study was undertaken to evaluate the safety, immunologic effects, and antiviral activity of peripheral lymphocytes transferred from an elite controller, whose immune system is able to control viral replication without antiretroviral medications, to an HLA-B*2705-matched progressor.METHODSApproximately 22 billion cells were collected from an elite controller by lymphapheresis and infused within 6 hours into a recipient with a preinfusion CD4+ T cell count of 10 cells/μL (1%) and HIV plasma viral load of 114,993 copies/mL.RESULTSDonor cells were cleared from the recipient's peripheral blood by day 8. A transient decrease in viral load to 58,421 (day 3) was followed by a rebound to 702,972 (day 6) before returning to baseline values by day 8. The decreased viral load was temporally associated with peak levels of donor T cells, including CD8+ T cells that had high levels of expression of Ki67, perforin, and granzyme B. Notably, recipient CD8+ T cells also showed increased expression of these markers, especially in HIV-specific tetramer-positive cells.CONCLUSIONThese results suggest that the adoptive transfer of lymphocytes from an HIV-infected elite controller to an HIV-infected patient with progressive disease may be able to perturb the immune system of the recipient in both positive and negative ways.TRIAL REGISTRATIONClinicalTrials.gov NCT00559416.FUNDINGIntramural Research Programs of the US NIH Clinical Center and the National Institute of Allergy and Infectious Diseases (NIAID); the National Cancer Institute.
Keywords: AIDS/HIV; Adaptive immunity; Immunotherapy; Infectious disease; T cells.
Conflict of interest statement
Figures
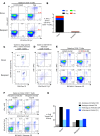
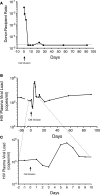
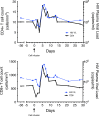
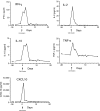

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

