Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: randomised, lansoprazole-controlled non-inferiority and single-blind extension study
- PMID: 28988197
- PMCID: PMC5969369
- DOI: 10.1136/gutjnl-2017-314010
Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: randomised, lansoprazole-controlled non-inferiority and single-blind extension study
Abstract
Objective: To assess the non-inferiority of vonoprazan to lansoprazole for secondary prevention of non-steroidal anti-inflammatory drug (NSAID)-induced peptic ulcer (PU) and the safety of vonoprazan during extended use.
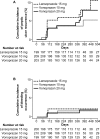
Design: A phase 3, 24-week, multicenter, randomised, double-blind (DB), active-controlled study, followed by a phase 3, ≥28 week, multicenter, single-blind, parallel-group extension study (EXT) in outpatients (n=642) receiving long-term NSAID therapy who are at risk of PU recurrence. The patients received vonoprazan (10 mg or 20 mg) or lansoprazole 15 mg once daily. For DB, non-inferiority of the proportion of patients with recurrent PU within 24 weeks was analysed by Farrington and Manning test (significance level 2.5%, non-inferiority margin 8.3%; primary endpoint), recurrent PU within 12 weeks, bleeding and time-to-event of PU (secondary endpoint) and treatment-emergent adverse events (TEAEs). For EXT, TEAEs (primary endpoint), recurrent PU and safety (secondary) were assessed up to 104 weeks for patients in the extension study.
Results: The non-inferiority of vonoprazan 10 mg and 20 mg to lansoprazole 15 mg was verified (percentage difference -2.2%,95% CI -6.2% to 1.8%, p<0.001; -2.1%,95% CI -6.1% to 2.0%, p<0.001, respectively). The proportion of patients with endoscopically confirmed recurrent PU within 24 weeks was 3.3%, 3.4% and 5.5%, for vonoprazan 10 mg, 20 mg and lansoprazole 15 mg, respectively. No significant safety concerns were identified.
Conclusion: The non-inferiority of vonoprazan (10 and 20 mg) was verified in patients receiving long-term NSAIDs in DB; it was effective and well tolerated in EXT for longer than 1 year, with a safety profile similar to lansoprazole (15 mg).
Trial registration numbers: NCT01452750, NCT01456260; Results.
Keywords: NSAIDs; lansoprazole osteoarthritis; non-inferiority; peptic ulcer; potassium-competitive acid blockers; rheumatoid arthritis; vonoprazan.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: YM has served as a consultant for, received grant and honorarium from Takeda Pharmaceutical Company. KO, NF and AN are employees of Takeda Pharmaceutical Company. SS has served as a consultant for and received a grant, honorarium and travel fee from Takeda Pharmaceutical Company. TK, KA and KS have served as a consultant and received a grant and honorarium from Takeda Pharmaceutical Company.
Figures



Comment in
-
Vonoprazan, aspirin and NSAIDs: new era in acid inhibition and gastroprotection.Gut. 2018 Jun;67(6):995-996. doi: 10.1136/gutjnl-2017-315252. Epub 2017 Dec 5. Gut. 2018. PMID: 29208676 No abstract available.
References
-
- Shiokawa Y, Nobunaga M, Saito T, et al. . [Epidemiology study on upper gastrointestinal lesions induced by non-steroidal anti-inflammatory drugs]. Ryumachi 1991;31:96–111. - PubMed
-
- Larkai EN, Smith JL, Lidsky MD, et al. . Gastroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic nonsteroidal anti-inflammatory drug use. Am J Gastroenterol 1987;82:1153–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
