Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells
- PMID: 27482888
- PMCID: PMC5004935
- DOI: 10.1172/JCI86721
Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells
Abstract
Background: T cells expressing antigen-specific chimeric antigen receptors (CARs) improve outcomes for CD19-expressing B cell malignancies. We evaluated a human application of T cells that were genetically modified using the Sleeping Beauty (SB) transposon/transposase system to express a CD19-specific CAR.
Methods: T cells were genetically modified using DNA plasmids from the SB platform to stably express a second-generation CD19-specific CAR and selectively propagated ex vivo with activating and propagating cells (AaPCs) and cytokines. Twenty-six patients with advanced non-Hodgkin lymphoma and acute lymphoblastic leukemia safely underwent hematopoietic stem cell transplantation (HSCT) and infusion of CAR T cells as adjuvant therapy in the autologous (n = 7) or allogeneic settings (n = 19).
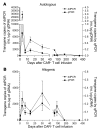
Results: SB-mediated genetic transposition and stimulation resulted in 2,200- to 2,500-fold ex vivo expansion of genetically modified T cells, with 84% CAR expression, and without integration hotspots. Following autologous HSCT, the 30-month progression-free and overall survivals were 83% and 100%, respectively. After allogeneic HSCT, the respective 12-month rates were 53% and 63%. No acute or late toxicities and no exacerbation of graft-versus-host disease were observed. Despite a low antigen burden and unsupportive recipient cytokine environment, CAR T cells persisted for an average of 201 days for autologous recipients and 51 days for allogeneic recipients.
Conclusions: CD19-specific CAR T cells generated with SB and AaPC platforms were safe, and may provide additional cancer control as planned infusions after HSCT. These results support further clinical development of this nonviral gene therapy approach.
Trial registration: Autologous, NCT00968760; allogeneic, NCT01497184; long-term follow-up, NCT01492036.
Funding: National Cancer Institute, private foundations, and institutional funds. Please see Acknowledgments for details.
Figures