Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial
- PMID: 27825638
- PMCID: PMC5154681
- DOI: 10.1016/S1470-2045(16)30561-7
Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial
Abstract
Background: Erlotinib is approved for the treatment of all patients with advanced non-small-cell lung cancer (NSCLC), but is most active in the treatment of EGFR mutant NSCLC. Cabozantinib, a small molecule tyrosine kinase inhibitor, targets MET, VEGFR, RET, ROS1, and AXL, which are implicated in lung cancer tumorigenesis. We compared the efficacy of cabozantinib alone or in combination with erlotinib versus erlotinib alone in patients with EGFR wild-type NSCLC.
Methods: This three group, randomised, controlled, open-label, multicentre, phase 2 trial was done in 37 academic and community oncology practices in the USA. Patients were eligible if they had received one or two previous treatments for advanced non-squamous, EGFR wild-type, NSCLC. Patients were stratified by performance status and line of therapy, and randomly assigned using permuted blocks within strata to receive open-label oral daily dosing of erlotinib (150 mg), cabozantinib (60 mg), or erlotinib (150 mg) and cabozantinib (40 mg). Imaging was done every 8 weeks. At the time of radiographic progression, there was optional crossover for patients in either single-drug group to receive combination treatment. The primary endpoint was to compare progression-free survival in patients given erlotinib alone versus cabozantinib alone, and in patients given erlotinib alone versus the combination of erlotinib plus cabozantinib. We assessed the primary endpoint in the per-protocol population, which was defined as all patients who were eligible, randomly assigned, and received at least one dose of treatment. The safety analysis population included all patients who received study treatment irrespective of eligibility. This trial is registered with ClinicalTrials.gov, number NCT01708954.
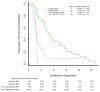
Findings: Between Feb 7, 2013, and July 1, 2014, we enrolled and randomly assigned 42 patients to erlotinib treatment, 40 patients to cabozantinib treatment, and 43 patients to erlotinib plus cabozantinib treatment, of whom 111 (89%) in total were included in the primary analysis (erlotinib [n=38], cabozantinib [n=38], erlotinib plus cabozantinib [n=35]). Compared with erlotinib alone (median 1·8 months [95% CI 1·7-2·2]), progression-free survival was significantly improved in the cabozantinib group (4·3 months [3·6-7·4]; hazard ratio [HR] 0·39, 80% CI 0·27-0·55; one-sided p=0·0003) and in the erlotinib plus cabozantinib group (4·7 months [2·4-7·4]; HR 0·37, 0·25-0·53; one-sided p=0·0003). Among participants included in the safety analysis of the erlotinib (n=40), cabozantinib (n=40), and erlotinib plus cabozantinib (n=39) groups, the most common grade 3 or 4 adverse events were diarrhoea (three [8%] cases in the erlotinib group vs three [8%] in the cabozantinib group vs 11 [28%] in the erlotinib plus cabozantinib group), hypertension (none vs ten [25%] vs one [3%]), fatigue (five [13%] vs six [15%] vs six [15%]), oral mucositis (none vs four [10%] vs one [3%]), and thromboembolic event (none vs three [8%] vs two [5%]). One death due to respiratory failure occurred in the cabozantinib group, deemed possibly related to either drug, and one death due to pneumonitis occurred in the erlotinib plus cabozantinib group, deemed related to either drug or the combination.
Interpretation: Despite its small sample size, this trial showed that, in patients with EGFR wild-type NSCLC, cabozantinib alone or combined with erlotinib has clinically meaningful, superior efficacy to that of erlotinib alone, with additional toxicity that was generally manageable. Cabozantinib-based regimens are promising for further investigation in this patient population.
Funding: ECOG-ACRIN Cancer Research Group, National Cancer Institute of the National Institutes of Health.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests Dr. Neal reports personal fees from Clovis, personal fees from CARET/Physicians Resource, grants and personal fees from Nektar, grants and personal fees from Boehringer Ingelheim, personal fees from ARMO BioSciences, grants from Genentech/Roche, grants from Merck, grants from ArQule, grants from Novartis, outside the submitted work. Dr. Dahlberg reports a patent PCT/US2014/054821 pending to Dana-Farber Cancer Institute. Dr. Wakelee reports personal fees from Peregrine, grants and personal fees from Helsinn, personal fees from ACEA, grants and personal fees from Pfizer, grants from BMS, grants from XCovery, grants from Celgene, grants and personal fees from Roche/Genentech, grants from AstraZeneca/MedImmune, personal fees from Lilly, grants from Gilead, grants from Clovis, grants from Pharmacyclics, grants from Exelixis, grants from Novartis, outside the submitted work. Dr. Carbone reports personal fees from Ariad, personal fees from AstraZeneca, personal fees from Bayer HealthCare, personal fees from Biothera, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers-Squibb, personal fees from Clovis Oncology, personal fees from Genentech/Roche, personal fees from Guardant Health, personal fees from Inivata, personal fees from Janssen Diagnostics, personal fees from Merck, personal fees from Novartis, personal fees from Peregrine Pharmaceuticals, personal fees from Synta Pharmaceuticals, personal fees from Teva Pharmaceuticals, outside the submitted work. Dr. Lerner reports grant from Acerta Pharma, grant from Amgen, grant from Bayer HealthCare Pharmaceuticals Inc., grant from Celcuity, LLC, grant from Cephalon, Inc., grant from Teva Branded Pharmaceutical Products R&D, Inc., grant from Janssen Research and Development, LLC, grant from Pfizer, grant from Roche/Genentech, Inc., outside the submitted work. Dr. Ramalingam reports personal fees from Amgen, AstraZeneca, Abbvie, BMS, Lilly, Celgene, Genentech, and Novartis outside the submitted work. The other authors declared no conflicts of interest.
Figures



Comment in
-
RET inhibitors for patients with RET fusion-positive and RET wild-type non-small-cell lung cancer.Lancet Oncol. 2016 Dec;17(12):1623-1625. doi: 10.1016/S1470-2045(16)30557-5. Epub 2016 Nov 4. Lancet Oncol. 2016. PMID: 27825637 No abstract available.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011 Mar-Apr;61(2):69–90. - PubMed
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002 Jan 10;346(2):92–8. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004 May 1;22(9):1589–97. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000 May;18(10):2095–103. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA035267/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- UG1 CA189825/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- U10 CA107868/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- U10 CA098413/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
- U10 CA180864/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

