Efficacy of mitomycin C in endoscopic dacryocystorhinostomy: a systematic review and meta-analysis
- PMID: 23675423
- PMCID: PMC3652813
- DOI: 10.1371/journal.pone.0062737
Efficacy of mitomycin C in endoscopic dacryocystorhinostomy: a systematic review and meta-analysis
Abstract
Background: A number of published comparative studies have been conducted to evaluate the efficacy and safety of intraoperative mitomycin C (MMC) in endoscopic dacryocystorhinostomy (EN-DCR). However, results have not always been consistent. Therefore, we carried out a meta-analysis to compare the clinical results of EN-DCR with and without MMC.
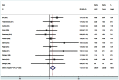
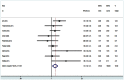
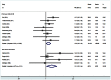
Methods and findings: A comprehensive literature search of Cochrane Library, PubMed and EMBASE to identify relevant trials comparing EN-DCR with and without MMC. Eleven studies including 574 eyes were included in this meta-analysis. The success was defined as patency of the nasolacrimal canal and symptomatic improvement. There was significantly higher success rate in the MMC group in comparison with control group [RR = 1.12, 95% CI (1.04, 1.20), P = 0.004]. A sensitivity analysis after the non-randomized controlled trials were excluded from the meta-analysis demonstrated no differences compared with the overall results. Subgroup analyses showed that MMC group had a significantly higher success rate than control group in primary and revision EN-DCR, and EN-DCR without silicone intubation, but no difference in the subgroup of with silicone intubation. The size of the osteotomy site was bigger in the MMC group compared to the control group at 3 months [WMD = 7.65, 95% CI (0.33, 14.98), P = 0.041] and 6 months [WMD = 9.28, 95% CI (2.45, 16.11), P = 0.008] after surgery. However, there was statistically significant difference in the osteotomy surface area between the two groups at 12 months after surgery [WMD = 11.63, 95% CI (-1.04, 24.29), P = 0.072].
Conclusion: Intraoperative MMC application seems to be a safe adjuvant that could reduce the closure rate of the osteotomy and enhance the success rate after both primary and revision EN-DCR.
Trial registration: ClinicalTrials.gov NCT01772277.
Conflict of interest statement
Figures


References
-
- Caldwell GW (1893) Two new operations for obstruction of the nasal duct with preservation of the canaliculi. Am J Ophthalmol 10: 189–191.
-
- McDonogh M, Meiring JH (1989) Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol 103: 585–587. - PubMed
-
- Korkut AY, Teker AM, Yazici MZ, Kahya V, Gedikli O, et al. (2010) Surgical outcomes of primary and revision endoscopic dacryocystorhinostomy. J Craniofac Surg 21: 1706–1708. - PubMed
-
- Leong SC, Macewen CJ, White PS (2010) A systematic review of outcomes after dacryocystorhinostomy in adults. Am J Rhinol Allergy 24: 81–90. - PubMed
-
- Wakaki S, Marumo H, Tomioka K (1958) Isolation of new fractions of antitumor mitomycins. Antibiot Chemother 8: 228–240. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

