Efficacy of Methotrexate Alone vs Methotrexate Plus Low-Dose Prednisone in Patients With Alopecia Areata Totalis or Universalis: A 2-Step Double-Blind Randomized Clinical Trial
- PMID: 36884234
- PMCID: PMC9996454
- DOI: 10.1001/jamadermatol.2022.6687
Efficacy of Methotrexate Alone vs Methotrexate Plus Low-Dose Prednisone in Patients With Alopecia Areata Totalis or Universalis: A 2-Step Double-Blind Randomized Clinical Trial
Abstract
Importance: Poor therapeutic results have been reported in patients with alopecia areata totalis (AT) or universalis (AU), the most severe and disabling types of alopecia areata (AA). Methotrexate, an inexpensive treatment, might be effective in AU and AT.
Objective: To evaluate the efficacy and tolerance of methotrexate alone or combined with low-dose prednisone in patients with chronic and recalcitrant AT and AU.
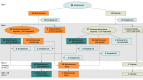
Design, setting, and participants: This academic, multicenter, double-blind, randomized clinical trial was conducted at 8 dermatology departments at university hospitals between March 2014 and December 2016 and included adult patients with AT or AU evolving for more than 6 months despite previous topical and systemic treatments. Data analysis was performed from October 2018 to June 2019.
Interventions: Patients were randomized to receive methotrexate (25 mg/wk) or placebo for 6 months. Patients with greater than 25% hair regrowth (HR) at month 6 continued their treatment until month 12. Patients with less than 25% HR were rerandomized: methotrexate plus prednisone (20 mg/d for 3 months and 15 mg/d for 3 months) or methotrexate plus placebo of prednisone.
Main outcome and measures: The primary end point assessed on photos by 4 international experts was complete or almost complete HR (Severity of Alopecia Tool [SALT] score <10) at month 12, while receiving methotrexate alone from the start of the study. Main secondary end points were the rate of major (greater than 50%) HR, quality of life, and treatment tolerance.
Results: A total of 89 patients (50 female, 39 male; mean [SD] age, 38.6 [14.3] years) with AT (n = 1) or AU (n = 88) were randomized: methotrexate (n = 45) or placebo (n = 44). At month 12, complete or almost complete HR (SALT score <10) was observed in 1 patient and no patient who received methotrexate alone or placebo, respectively, in 7 of 35 (20.0%; 95% CI, 8.4%-37.0%) patients who received methotrexate (for 6 or 12 months) plus prednisone, including 5 of 16 (31.2%; 95% CI, 11.0%-58.7%) who received methotrexate for 12 months and prednisone for 6 months. A greater improvement in quality of life was observed in patients who achieved a complete response compared with nonresponder patients. Two patients in the methotrexate group discontinued the study because of fatigue and nausea, which were observed in 7 (6.9%) and 14 (13.7%) patients receiving methotrexate, respectively. No severe treatment adverse effect was observed.
Conclusions and relevance: In this randomized clinical trial, while methotrexate alone mainly allowed partial HR in patients with chronic AT or AU, its combination with low-dose prednisone allowed complete HR in up to 31% of patients. These results seem to be of the same order of magnitude as those recently reported with JAK inhibitors, with a much lower cost.
Trial registration: ClinicalTrials.gov Identifier: NCT02037191.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

