Torque teno virus viremia and QuantiFERON®-CMV assay in prediction of cytomegalovirus reactivation in R+ kidney transplant recipients
- PMID: 37425298
- PMCID: PMC10323437
- DOI: 10.3389/fmed.2023.1180769
Torque teno virus viremia and QuantiFERON®-CMV assay in prediction of cytomegalovirus reactivation in R+ kidney transplant recipients
Abstract
Introduction: Cytomegalovirus (CMV) is the most frequent infectious complication following solid organ transplantation. Torque teno viruses (TTV) viremia has been proposed as a biomarker of functional immunity in the management of kidney transplant recipients (KTR). The QuantiFERON®-CMV (QF-CMV) is a commercially available assay that allows the assessment of CD8+ T-cell responses in routine diagnostic laboratories.
Methods: In a prospective national multicenter cohort of 64 CMV-seropositive (R+) KTR, we analyzed the value of TTV load and the two markers of the QF-CMV assay [QF-Ag (CMV-specific T-cell responses) and QF-Mg (overall T-cell responses)], alone and in combination, in prediction of CMV reactivation (≥3 log10 IU/ ml) in the first post-transplant year. We compared previously published cut-offs and specific cut-offs optimized from ROC curves for our population.
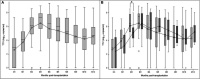
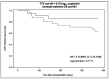
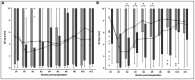
Results: Using the conventional cut-off (3.45 log10 copies/ml), TTV load at D0 [inclusion visit on the day of transplantation before induction (D0)], or at M1 (1-month post-transplant visit) perform better in predicting CMV viremia control than CMV reactivation. Survival analyses suggest a better performance of our optimized TTV cut-offs (3.78 log10 copies/ml at D0 and 4.23 log10 copies/ml at M1) for risk stratification of CMV reactivation in our R+ KTR cohort. The QF-CMV (QF-Ag = 0.2 IU/ml, and QF-Mg = 0.5 IU/ml) also appears to better predict CMV viremia control than CMV reactivation. Moreover, survival analyses suggest that the QF-Mg would perform better than the QF-Ag in stratifying the risk of CMV reactivation. The use of our optimized QF-Mg cut-off (1.27 IU/ml) at M1 further improved risk stratification of CMV reactivation. Using conventional cut-offs, the combination of TTV load and QF-Ag or TTV load and QF-Mg did not improve prediction of CMV viremia control compared to separate analysis of each marker but resulted in an increase of positive predictive values. The use of our cut-offs slightly improved risk prediction of CMV reactivation.
Conclusion: The combination of TTV load and QF-Ag or TTV load and QF-Mg could be useful in stratifying the risk of CMV reactivation in R+ KTR during the first post-transplant year and thereby have an impact on the duration of prophylaxis in these patients.
Clinical trial registration: ClinicalTrials.gov registry, identifier NCT02064699.
Keywords: CMV-seropositive recipients; QuantiFERON® CMV; Torquetenovirus; cytomegalovirus; kidney transplantation.
Copyright © 2023 Mafi, Essig, Rerolle, Lagathu, Crochette, Brodard, Schvartz, Gouarin, Bouvier, Engelmann, Garstka, Bressollette-Bodin, Cantarovitch, Germi, Janbon, Archimbaut, Heng, Garnier, Gomes-Mayeras, Labrunie, Hantz and Alain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

