Use of Auto-Injector for Methotrexate Subcutaneous Self-Injections: High Satisfaction Level and Good Compliance in SELF-I Study, a Randomized, Open-Label, Parallel Group Study
- PMID: 30547379
- PMCID: PMC6393262
- DOI: 10.1007/s40744-018-0134-2
Use of Auto-Injector for Methotrexate Subcutaneous Self-Injections: High Satisfaction Level and Good Compliance in SELF-I Study, a Randomized, Open-Label, Parallel Group Study
Abstract
Introduction: The objective of the study was to compare compliance and acceptability of a new auto-injector (AI) versus syringe for administration of methotrexate (MTX) in patients with rheumatoid arthritis (RA).
Methods: We conducted a randomized, open-label, parallel group study comparing AI to pre-filled syringe (PFS). Adult patients with RA (ACR/EULAR 2010) receiving MTX (orally or by injection) for at least 3 months were allocated to AI or PFS for 6 months and then were allocated to AI for 6 further months. Two co-primary endpoints were defined at M6: percentage of patients with compliance at least 80%; change in functional capacity assessed by Health Assessment Questionnaire (HAQ). Secondary endpoints included quality of life (RaQoL), RA activity (DAS28), and acceptability. Local safety at injection site was assessed at each visit.
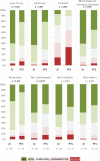
Results: Two-hundred and sixty-five patients were randomized. The main analysis was conducted on per protocol set (99 AI and 98 PFS). Compliance was 96.2% in AI and 98.9% in PFS. Good complier rates were 89.9% and 94.9%, thus a difference of - 5.0% (- 18.9%; 8.9%). HAQ remained stable in both groups. No difference was found on RaQoL, change in RA activity, and safety profile. Autonomy, acceptability, and patient satisfaction were better with AI, and patients having had the experience of both AI and PFS preferred AI (p < 0.001).
Conclusions: Although this study did not demonstrate non-inferiority of AI versus PFS, compliance was excellent in the two groups, and AI, which was preferred by patients, is a valuable alternative to PFS for administration of MTX.
Trial registration: ClinicalTrials.gov identifier, NCT02553018.
Funding: Nordic Pharma SAS.
Keywords: Auto-injector; Compliance; Methotrexate; Rheumatoid arthritis; Satisfaction.
Figures

References
-
- Jacobs JW. Lessons for the use of non-biologic anchor treatments for rheumatoid arthritis in the era of biologic therapies. Rheumatology. 2012;51(suppl_4):iv27–iv33. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

