Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials
- PMID: 29217375
- PMCID: PMC5884730
- DOI: 10.1016/S0140-6736(17)33106-9
Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials
Erratum in
-
Department of Error.Lancet. 2020 Jun 20;395(10241):1906. doi: 10.1016/S0140-6736(19)31427-8. Lancet. 2020. PMID: 32563375 No abstract available.
Abstract
Background: A safe, effective, and rapidly scalable vaccine against Zika virus infection is needed. We developed a purified formalin-inactivated Zika virus vaccine (ZPIV) candidate that showed protection in mice and non-human primates against viraemia after Zika virus challenge. Here we present the preliminary results in human beings.
Methods: We did three phase 1, placebo-controlled, double-blind trials of ZPIV with aluminium hydroxide adjuvant. In all three studies, healthy adults were randomly assigned by a computer-generated list to receive 5 μg ZPIV or saline placebo, in a ratio of 4:1 at Walter Reed Army Institute of Research, Silver Spring, MD, USA, or of 5:1 at Saint Louis University, Saint Louis, MO, USA, and Beth Israel Deaconess Medical Center, Boston, MA, USA. Vaccinations were given intramuscularly on days 1 and 29. The primary objective was safety and immunogenicity of the ZPIV candidate. We recorded adverse events and Zika virus envelope microneutralisation titres up to day 57. These trials are registered at ClinicalTrials.gov, numbers NCT02963909, NCT02952833, and NCT02937233.
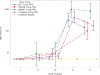
Findings: We enrolled 68 participants between Nov 7, 2016, and Jan 25, 2017. One was excluded and 67 participants received two injections of Zika vaccine (n=55) or placebo (n=12). The vaccine caused only mild to moderate adverse events. The most frequent local effects were pain (n=40 [60%]) or tenderness (n=32 [47%]) at the injection site, and the most frequent systemic reactogenic events were fatigue (29 [43%]), headache (26 [39%]), and malaise (15 [22%]). By day 57, 52 (92%) of vaccine recipients had seroconverted (microneutralisation titre ≥1:10), with peak geometric mean titres seen at day 43 and exceeding protective thresholds seen in animal studies.
Interpretation: The ZPIV candidate was well tolerated and elicited robust neutralising antibody titres in healthy adults.
Funding: Departments of the Army and Defense and National Institute of Allergy and Infectious Diseases.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the other authors have any disclosures or conflicts of interest to report.
Figures




Comment in
-
Tradition and innovation in development of a Zika vaccine.Lancet. 2018 Feb 10;391(10120):516-517. doi: 10.1016/S0140-6736(17)33107-0. Epub 2017 Dec 5. Lancet. 2018. PMID: 29217377 No abstract available.
References
-
- Honein MA, Dawson AL, Petersen EE, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2017;317:59–68. - PubMed
-
- Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016;73:1407–16. - PubMed
-
- Mlakar J, Korva M, Tul N, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN272201200003I/AI/NIAID NIH HHS/United States
- I01 BX003714/BX/BLRD VA/United States
- HHSN27200007/AO/NIAID NIH HHS/United States
- K22 AI104794/AI/NIAID NIH HHS/United States
- HHSN272201300021C/AI/NIAID NIH HHS/United States
- HHSN272201000021I/AI/NIAID NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- UL1 TR000170/TR/NCATS NIH HHS/United States
- K23 AI114381/AI/NIAID NIH HHS/United States
- HHSN272201000021C/AI/NIAID NIH HHS/United States
- R21 MH112360/MH/NIMH NIH HHS/United States
- HHSN27200020/AO/NIAID NIH HHS/United States
- HHSN272201300021I/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

