Strain-resolved analysis in a randomized trial of antibiotic pretreatment and maintenance dose delivery mode with fecal microbiota transplant for ulcerative colitis
- PMID: 35365713
- PMCID: PMC8976058
- DOI: 10.1038/s41598-022-09307-5
Strain-resolved analysis in a randomized trial of antibiotic pretreatment and maintenance dose delivery mode with fecal microbiota transplant for ulcerative colitis
Abstract
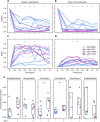
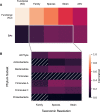
Fecal microbiota transplant is a promising therapy for ulcerative colitis. Parameters maximizing effectiveness and tolerability are not yet clear, and it is not known how import the transmission of donor microbes to patients is. Here (clinicaltrails.gov: NCT03006809) we have tested the effects of antibiotic pretreatment and compared two modes of maintenance dose delivery, capsules versus enema, in a randomized, pilot, open-label, 2 × 2 factorial design with 22 patients analyzed with mild to moderate UC. Clinically, the treatment was well-tolerated with favorable safety profile. Of patients who received antibiotic pretreatment, 6 of 11 experienced remission after 6 weeks of treatment, versus 2 of 11 non-pretreated patients (log odds ratio: 1.69, 95% confidence interval: -0.25 to 3.62). No significant differences were found between maintenance dosing via capsules versus enema. In exploratory analyses, microbiome turnover at both the species and strain levels was extensive and significantly more pronounced in the pretreated patients. Associations were also revealed between taxonomic turnover and changes in the composition of primary and secondary bile acids. Together these findings suggest that antibiotic pretreatment contributes to microbiome engraftment and possibly clinical effectiveness, and validate longitudinal strain tracking as a powerful way to monitor the dynamics and impact of microbiota transfer.
© 2022. The Author(s).
Conflict of interest statement
YMP is an employee of Symbiome, Inc. ZK is an employee/shareholder at Finch Therapeutics. SVL is co-founder and shareholder of Siolta Therapeutics, Inc. and serves as both a consultant and a member of its Board of Directors. KSP is on the scientific advisory board of Phylagen. NE has received research support from Finch Therapeutics and Assembly Biosciences, and has been a consultant for Federation Bio and Ferring Pharmaceuticals. All other authors declare no competing interests.
Figures


References
-
- Lopetuso LR, et al. Fecal transplantation for ulcerative colitis: Current evidence and future applications. Expert Opin. Biol. Ther. 2020;20:343–351. - PubMed
-
- Paramsothy S, et al. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohns Colitis. 2017;11:1180–1199. - PubMed
-
- Costello SP, et al. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 2017;46:213–224. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

