Impact of N-Acetyl-Cysteine on Ischemic Stumps Following Major Lower Extremity Amputation: A Pilot Randomized Clinical Trial
- PMID: 35129469
- PMCID: PMC9987417
- DOI: 10.1097/SLA.0000000000005389
Impact of N-Acetyl-Cysteine on Ischemic Stumps Following Major Lower Extremity Amputation: A Pilot Randomized Clinical Trial
Abstract
Objective: To evaluate the impact of N-acetyl-cysteine (NAC) on amputation stump perfusion and healing in patients with critical limb-threatening ischemia (CLTI).
Background: Patients with CLTI are at increased risk of poor amputation site healing leading to increased procedure-associated morbidity.
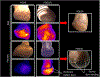
Methods: In a pilot, double-blind, placebo-controlled, randomized controlled trial, patients with CLTI undergoing major elective lower extremity amputation were randomized 1:1 to intravenous NAC (1200 mg twice-daily) or placebo for up to 5 days postoperatively. Primary outcomes were change in stump perfusion at postoperative day 3 (POD3) and POD5, and healing at POD30. Stumps were serially evaluated for wound healing, and tissue perfusion was evaluated using noninvasive laser-assisted fluorescent angiography.
Results: Thirty-three patients were randomized to NAC (n = 16) or placebo (n = 17). Thirty-one patients were eligible for intent-to-treat analysis (NAC14; placebo17). Twenty patients (NAC7; placebo13) had amputation stump perfusion defects at POD0 and were considered high-risk for poor healing. Intent-to-treat analysis revealed no significant differences between treatment groups. Subgroup analysis of high-risk patients revealed differences in stump perfusion defect size (NAC-0.53-fold, placebo +0.71-fold; 95% confidence interval -2.11 to-0.35; P < 0.05) and healing (NAC [100%], placebo [46%]; P < 0.01) between study treatments.
Conclusions: Postoperative NAC administration may improve amputation stump perfusion and healing in patients with CLTI and tissue perfusion defects at the time of amputation. Intraoperative laser-assisted fluorescent angiogra-phy may help surgeons identify high-risk patients with stump perfusion defects and provide early adjunctive interventions. Future studies can further explore the therapeutic benefits of NAC in the healing and perfusion of other surgical operative sites in high-risk individuals.
Trial registration: clinicaltrials.gov, Identifier: NCT03253328.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

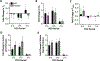

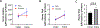
References
-
- Zayed M, Bech F, Hernandez-Boussard T. National review of factors influencing disparities and types of major lower extremity amputations. Ann Vasc Surg 2014; 28(5):1157–65. - PubMed
-
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil 2005; 86(3):480–6. - PubMed
-
- Ebskov B, Josephsen P. Incidence of reamputation and death after gangrene of the lower extremity. Prosthet Orthot Int 1980; 4(2):77–80. - PubMed
-
- Pourghaderi P, Yuquimpo KM, Roginski Guetter C, et al. Outcomes following Lower Extremity Amputation in Patients with Diabetes Mellitus and Peripheral Arterial Disease. Ann Vasc Surg 2020; 63:259–268. - PubMed

