Evaluation of Six Weekly Oral Fecal Microbiota Transplants in People with HIV
- PMID: 33501400
- PMCID: PMC7815055
- DOI: 10.20411/pai.v5i1.388
Evaluation of Six Weekly Oral Fecal Microbiota Transplants in People with HIV
Abstract
Background: Reduced microbiota diversity (dysbiosis) in people with HIV (PWH) likely contributes to inflammation, a driver of morbidity and mortality. We aimed to evaluate the safety and tolerability of 6 weekly oral fecal microbiota transplants (FMT) administered to reverse this dysbiosis.
Methods: Six PWH on suppressive antiretroviral therapy (ART) received 6 weekly doses of lyophilized fecal microbiota product from healthy donors. Shotgun sequencing on stool before, after last FMT, and 20 weeks thereafter was performed. Inflammation and gut permeability biomarkers were measured.
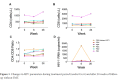
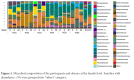
Results: Median age at week 0 was 39 years, CD4+ T cell count 496 cells/mm3, HIV RNA levels <20 copies/mL. FMT was safe and well-tolerated. α diversity increased in 4 participants from weeks 0 to 6, including the 3 with the lowest α diversity at week 0. At week 26, α diversity more closely resembled week 0 than week 6 in these 4 participants. Metagenomic analysis showed no consistent changes across all participants. One participant had high gut permeability and inflammation biomarker levels and low α diversity that improved between weeks 0 and 6 with a shift in distribution.
Conclusions: Weekly FMT was safe and well-tolerated. α diversity increased in participants with the lowest baseline α diversity during the treatment period. Future randomized, controlled trials of FMT should consider evaluating PWH with greater inflammation, gut damage, or dysbiosis as this population may be most likely to show a significant response.ClinicalTrials.gov Identifier: NCT03329560.
Keywords: HIV; fecal microbiota transplant; inflammation; microbiome.
Copyright © Pathogens and Immunity 2020.
Conflict of interest statement
H.L.D. and Z-D.J. have applied for a patent for PRIM-DJ2727, and H.L.D. has received a grant from Rebiotix to study their FMT product. MF is an employee of Associates of Cape Cod, Inc., the manufacturer of the (1,3)-β-D-glucan test used in this study. No other authors have competing interests.
Figures



References
-
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–90. Epub 2011/January/22. doi: 10.1093/infdis/jiq118. PubMed PMID: 21252259; PMCID: Pmc3071127. - DOI - PMC - PubMed
-
- Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. Epub 2014/May/06. doi: 10.1093/infdis/jiu254. PubMed PMID: 24795473; PMCID: PMC4192039. - DOI - PMC - PubMed
-
- Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228–38. Epub 2014/April/24. doi: 10.1093/infdis/jiu238. PubMed PMID: 24755434; PMCID: PMC4192038. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
