A Novel Supraglottic Suction Device in Mechanically Ventilated Patients: A Randomized Controlled Trial
- PMID: 40161252
- PMCID: PMC11952068
- DOI: 10.2147/MDER.S499924
A Novel Supraglottic Suction Device in Mechanically Ventilated Patients: A Randomized Controlled Trial
Abstract
Objective: To evaluate the efficacy and safety of the SUPRAtube innovation device in preventing ventilator associated events and fluid accumulation in the supraglottic region in patients receiving mechanical ventilation (MV) through orotracheal tubes.
Methods: Multicenter, controlled, randomized, parallel, open-label clinical trial with a 1:1 allocation ratio of MV patients compared the use of the SUPRAtube elastomeric device with standard care and aspiration techniques. A series of computer-random numbers and centralized allocation with sealed envelopes were used.
Setting: Adult patients (n=108; mean age: 63 yrs, range: 19-85) hospitalized in intensive care units of two centers, the Cardiovascular Foundation of Colombia and the International Hospital of Colombia (Santander, Colombia), were included. All patients received MV through orotracheal tubes, were hemodynamically stable, had upper airway integrity according to fiberoptic bronchoscope findings, and had basic coagulation tests within acceptable risk criteria.
Interventions: Comprehensive standard of care, including preventive strategies, medical therapy, positive pressure MV, and routine procedures for management of oropharyngeal and pulmonary secretions (humidification, patient mobilization, and airway suctioning), was compared with the standard of care plus continuous supraglottic suction with the new SUPRAtube device.
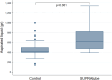
Results: The study period reached five days before extubation (media 85±7 hours). The weight of the aspirated content was 415 g (P25;P75: 396;536) in the control group and 624 g (P25;P75: 469;824) in the SUPRAtube group (p<0.001), equivalent to a mean difference of 213 g (P25;P75: 55;569; +50%). The device did not induce adverse events.
Conclusion: Continuous supraglottic aspiration using SUPRAtube is complementary, effective, safe, simple, and inexpensive and reduces the accumulation of oropharyngeal secretions in mechanically ventilated patients. The relevant clinical benefit in terms of preventing and improving tracheobronchitis earlier on was demonstrated by sequential fiberoptic bronchoscopy.
Registration in clinical trials: The present study is registered at clinicaltrials.gov NCT03573609.
Keywords: critical care; intratracheal intubation; mechanical ventilation; pneumonia; salivation; suction.
© 2025 Orozco-Levi et al.
Conflict of interest statement
Dr Mauricio Orozco-Levi reports a patent SUPRAtube. This device has received a patent as a utility model licensed to (ref. NC2016/0002059 Resolution 466). Dr Alba Ramírez-Sarmiento reports a patent NC2016/0002059 Dispositivo médico de aspiración supraglótica en pacientes ventilados mecánicamente licensed to Resolution 466 of the Superintendence of Industry and Commerce, Colombia. The authors report no other conflicts of interest in this work. An abstract of this paper was presented at the ERS Congress 2019 (Madrid, Spain) - Late Breaking Abstract conference talk, with interim findings. The abstract was published in the European Respiratory Journal 2019, with DOI https://doi.org/10.1183/13993003.congress-2019.RCT5098.
Figures


References
-
- Timsit JF, Schwebel C, Styfalova L, et al. Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med. 2019;45(6):834–843. PMID: 31020361. doi:10.1007/s00134-019-05622-0 - DOI - PubMed
-
- Ekren PK, Ranzani OT, Ceccato A, et al. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society guideline criteria for risk of multidrug-resistant pathogens in patients with hospital-acquired and ventilator-associated pneumonia in the ICU. Am J Respir Crit Care Med. 2018;197(6):826–830. PMID: 28902529. doi:10.1164/rccm.201708-1717LE - DOI - PubMed
-
- Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50(6):725–739;discussion739–741. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

