Reduction in vaso-occlusive events following stem cell transplantation in patients with sickle cell disease
- PMID: 36240296
- PMCID: PMC9860452
- DOI: 10.1182/bloodadvances.2022008137
Reduction in vaso-occlusive events following stem cell transplantation in patients with sickle cell disease
Abstract
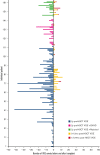
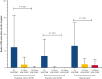
Hematopoietic stem cell transplantation (HSCT) is potentially curative for patients with sickle cell disease (SCD). Patients with stable donor engraftment after allogeneic HSCT generally do not experience SCD-related complications; however, there are no published data specifically reporting the change in vaso-occlusive events (VOE) after HSCT. Data regarding the number of VOEs requiring medical attention in the 2 years before allogeneic HSCT were compared with the number of VOEs in the 2 years (0-12 months and 12-24 months) after allogeneic HSCT in patients with SCD. One-hundred sixty-three patients with SCD underwent allogeneic HSCT between 2005 and 2019. The average age at the time of HSCT was 21 years (range, 7 months - 64 years). Most patients underwent nonmyeloablative conditioning (75% [N = 123]) and had a matched sibling donor (72% [N = 118]). The mean number of VOEs was reduced from 5.6 (range, 0-52) in the 2 years before HSCT to 0.9 (range, 0-12) in the 2 years after HSCT (P < .001). Among the post-HSCT events, VOE was more frequent during the first 12 months (0.8 [range, 0-12]) than at 12 to 24 months after HSCT (0.1 [range, 0-8) (P < .001)). In patients who had graft rejection (12%, N = 20), VOEs were reduced from 6.6 (range, 0-24) before HSCT to 1.1 (range, 0-6) and 0.8 (range, 0-8) at 0 to 12 months and 12 to 24 months after HSCT, respectively (P < .001). VOEs requiring medical care were significantly reduced after allogeneic HSCT for patients with SCD. These data will inform the development of novel autologous HSCT gene therapy approaches.
Trial registration: ClinicalTrials.gov NCT00061568 NCT02105766 NCT03077542 NCT00977691 NCT02165007 NCT03587272 NCT00745420 NCT02766465 NCT03263559 NCT04018937 NCT00029380.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.A. served on the safety monitoring committee for Sangamo Therapeutics. The remaining authors declare no competing financial interests.
Figures


References
-
- Shenoy S, Angelucci E, Arnold SD, et al. Current results and future research priorities in late effects after hematopoietic stem cell transplantation for children with sickle cell disease and thalassemia: a consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on late effects after pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(4):552–561. - PubMed
-
- Shenoy S, Gaziev J, Angelucci E, et al. Late effects screening guidelines after hematopoietic cell transplantation (HCT) for hemoglobinopathy: consensus statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on late effects after pediatric HCT. Biol Blood Marrow Transplant. 2018;24(7):1313–1321. - PubMed

