A randomized phase 3 trial to assess the immunogenicity and safety of 3 consecutively produced lots of freeze-dried MVA-BN® vaccine in healthy adults
- PMID: 36460535
- PMCID: PMC9707699
- DOI: 10.1016/j.vaccine.2022.10.056
A randomized phase 3 trial to assess the immunogenicity and safety of 3 consecutively produced lots of freeze-dried MVA-BN® vaccine in healthy adults
Abstract
Since vaccination remains the only effective protection against orthopox virus-induced diseases such as smallpox or monkeypox, the strategic use and stockpiling of these vaccines remains of significant public health importance. The approved liquid-frozen formulation of Bavarian Nordic's Modified Vaccinia Ankara (MVA-BN) smallpox vaccine has specific cold-chain requirements, while the freeze-dried (FD) formulation of this vaccine provides more flexibility in terms of storage conditions and shelf life. In this randomized phase 3 trial, the immunogenicity and safety of 3 consecutively manufactured lots of the FD MVA-BN vaccine was evaluated. A total of 1129 healthy adults were randomized to 3 treatment groups (lots 1 to 3) and received 2 vaccinations 4 weeks apart. For both neutralizing and total antibodies, a robust increase of geometric mean titer (GMT) was observed across all lot groups 2 weeks following the second vaccination, comparable to published data. For the primary results, the ratios of the neutralizing antibody GMTs between the lot group pairs ranged from 0.936 to 1.115, with confidence ratios well within the pre-specified margin of equivalence. Results for total antibodies were similar. In addition, seroconversion rates were high across the 3 lots, ranging between 99.1 % and 99.7 %. No safety concerns were identified; particularly, no inflammatory cardiac disorders were detected. The most common local solicited adverse events (AEs) reported across lot groups were injection site pain (87.2%) and erythema (73.2%), while the most common general solicited adverse events were myalgia, fatigue, and headache in 40.6% to 45.5% of all participants, with no meaningful differences among the lot groups. No related serious AEs were reported. In conclusion, the data demonstrate consistent and robust immunogenicity and safety results with a freeze-dried formulation of MVA-BN. Clinical Trial Registry Number: NCT03699124.
Keywords: Freeze-dried; Lot consistency; MVA-BN; Modified vaccinia Ankara; Monkeypox; Orthopoxvirus; Smallpox; Vaccine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
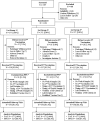

References
-
- Fenner F. Smallpox: emergence, global spread, and eradication. Hist Philos Life Sci. 1993;15(3):397–420. - PubMed
-
- Petersen B.W., Harms T.J., Reynolds M.G., Harrison L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses - Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257–262. doi: 10.15585/mmwr.mm6510a2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

