Traditional Chinese Medicine Compound (Tongxinluo) and Clinical Outcomes of Patients With Acute Myocardial Infarction: The CTS-AMI Randomized Clinical Trial
- PMID: 37874574
- PMCID: PMC10599127
- DOI: 10.1001/jama.2023.19524
Traditional Chinese Medicine Compound (Tongxinluo) and Clinical Outcomes of Patients With Acute Myocardial Infarction: The CTS-AMI Randomized Clinical Trial
Abstract
Importance: Tongxinluo, a traditional Chinese medicine compound, has shown promise in in vitro, animal, and small human studies for myocardial infarction, but has not been rigorously evaluated in large randomized clinical trials.
Objective: To investigate whether Tongxinluo could improve clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI).
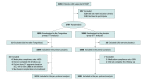
Design, setting, and participants: Randomized, double-blind, placebo-controlled clinical trial was conducted among patients with STEMI within 24 hours of symptom onset from 124 hospitals in China. Patients were enrolled from May 2019 to December 2020; the last date of follow-up was December 15, 2021.
Interventions: Patients were randomized 1:1 to receive either Tongxinluo or placebo orally for 12 months (a loading dose of 2.08 g after randomization, followed by the maintenance dose of 1.04 g, 3 times a day), in addition to STEMI guideline-directed treatments.
Main outcomes and measures: The primary end point was 30-day major adverse cardiac and cerebrovascular events (MACCEs), a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke. Follow-up for MACCEs occurred every 3 months to 1 year.
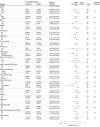
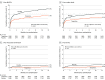
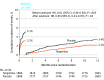
Results: Among 3797 patients who were randomized, 3777 (Tongxinluo: 1889 and placebo: 1888; mean age, 61 years; 76.9% male) were included in the primary analysis. Thirty-day MACCEs occurred in 64 patients (3.4%) in the Tongxinluo group vs 99 patients (5.2%) in the control group (relative risk [RR], 0.64 [95% CI, 0.47 to 0.88]; risk difference [RD], -1.8% [95% CI, -3.2% to -0.6%]). Individual components of 30-day MACCEs, including cardiac death (56 [3.0%] vs 80 [4.2%]; RR, 0.70 [95% CI, 0.50 to 0.99]; RD, -1.2% [95% CI, -2.5% to -0.1%]), were also significantly lower in the Tongxinluo group than the placebo group. By 1 year, the Tongxinluo group continued to have lower rates of MACCEs (100 [5.3%] vs 157 [8.3%]; HR, 0.64 [95% CI, 0.49 to 0.82]; RD, -3.0% [95% CI, -4.6% to -1.4%]) and cardiac death (85 [4.5%] vs 116 [6.1%]; HR, 0.73 [95% CI, 0.55 to 0.97]; RD, -1.6% [95% CI, -3.1% to -0.2%]). There were no significant differences in other secondary end points including 30-day stroke; major bleeding at 30 days and 1 year; 1-year all-cause mortality; and in-stent thrombosis (<24 hours; 1-30 days; 1-12 months). More adverse drug reactions occurred in the Tongxinluo group than the placebo group (40 [2.1%] vs 21 [1.1%]; P = .02), mainly driven by gastrointestinal symptoms.
Conclusions and relevance: In patients with STEMI, the Chinese patent medicine Tongxinluo, as an adjunctive therapy in addition to STEMI guideline-directed treatments, significantly improved both 30-day and 1-year clinical outcomes. Further research is needed to determine the mechanism of action of Tongxinluo in STEMI.
Trial registration: ClinicalTrials.gov Identifier: NCT03792035.
Conflict of interest statement
Figures
Comment in
-
Traditional Chinese Medicine Meets Evidence-Based Medicine in the Acutely Infarcted Heart.JAMA. 2023 Oct 24;330(16):1529-1530. doi: 10.1001/jama.2023.20838. JAMA. 2023. PMID: 37874582 No abstract available.
-
Traditional Chinese Medicine Compound and Clinical Outcomes in Acute Myocardial Infarction.JAMA. 2024 Apr 16;331(15):1332. doi: 10.1001/jama.2024.0620. JAMA. 2024. PMID: 38506843 No abstract available.
Similar articles
-
A review regarding the article 'Traditional Chinese medicine compound (Tongxinluo) and clinical outcomes of patients with acute myocardial infarction the CTS-AMI randomized clinical trial'.Curr Probl Cardiol. 2024 Sep;49(9):102692. doi: 10.1016/j.cpcardiol.2024.102692. Epub 2024 Jun 7. Curr Probl Cardiol. 2024. PMID: 38852911 Review.
-
China Tongxinluo Study for myocardial protection in patients with Acute Myocardial Infarction (CTS-AMI): Rationale and design of a randomized, double-blind, placebo-controlled, multicenter clinical trial.Am Heart J. 2020 Sep;227:47-55. doi: 10.1016/j.ahj.2020.06.011. Epub 2020 Jun 20. Am Heart J. 2020. PMID: 32679281 Free PMC article.
-
A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the effect of Tongmai Yangxin pill on ventricular remodeling in acute anterior STEMI patients after primary PCI.Phytomedicine. 2024 Dec;135:156133. doi: 10.1016/j.phymed.2024.156133. Epub 2024 Oct 17. Phytomedicine. 2024. PMID: 39489990 Clinical Trial.
-
Tongxinluo and Functional Outcomes Among Patients With Acute Ischemic Stroke: A Randomized Clinical Trial.JAMA Netw Open. 2024 Sep 3;7(9):e2433463. doi: 10.1001/jamanetworkopen.2024.33463. JAMA Netw Open. 2024. PMID: 39325453 Free PMC article. Clinical Trial.
-
Tongxinluo capsule as supplementation and cardiovascular endpoint events in patients with coronary heart disease: A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials.J Ethnopharmacol. 2022 May 10;289:115033. doi: 10.1016/j.jep.2022.115033. Epub 2022 Jan 26. J Ethnopharmacol. 2022. PMID: 35091010
Cited by
-
Translational Research and Clinical Application of Traditional Chinese Medicine in Cardiovascular Diseases.JACC Asia. 2024 Sep 17;4(10):711-720. doi: 10.1016/j.jacasi.2024.07.012. eCollection 2024 Oct. JACC Asia. 2024. PMID: 39553906 Free PMC article. Review.
-
Yiqi Wenyang decoction protects against the development of atherosclerosis by inhibiting vascular inflammation.Pharm Biol. 2025 Dec;63(1):264-274. doi: 10.1080/13880209.2025.2492650. Epub 2025 Apr 20. Pharm Biol. 2025. PMID: 40254717 Free PMC article.
-
From traditional medicine to modern medicine: the importance of TCM regulatory science (TCMRS) as an emerging discipline.Chin Med. 2025 Jun 26;20(1):92. doi: 10.1186/s13020-025-01152-8. Chin Med. 2025. PMID: 40563101 Free PMC article. Review.
-
Attitude, knowledge, and barriers of Chinese clinical and nursing students in implementing complementary and alternative medicine for COVID-19:a cross-sectional study.Heliyon. 2024 May 9;10(10):e30915. doi: 10.1016/j.heliyon.2024.e30915. eCollection 2024 May 30. Heliyon. 2024. PMID: 38778948 Free PMC article.
-
Effectiveness and mechanism of Huoxin pill on heart failure after percutaneous coronary intervention: Study protocol for a double-blind, randomised, placebo-controlled parallel trial.Contemp Clin Trials Commun. 2024 Jun 19;40:101328. doi: 10.1016/j.conctc.2024.101328. eCollection 2024 Aug. Contemp Clin Trials Commun. 2024. PMID: 39026569 Free PMC article.
References
-
- Hillerson D, Li S, Misumida N, et al. . Characteristics, process metrics, and outcomes among patients with ST-elevation myocardial infarction in rural vs urban areas in the US: a report from the US National Cardiovascular Data Registry. JAMA Cardiol. 2022;7(10):1016-1024. doi:10.1001/jamacardio.2022.2774 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

