Cilofexor in Patients With Compensated Cirrhosis Due to Primary Sclerosing Cholangitis: An Open-Label Phase 1B Study
- PMID: 38976363
- PMCID: PMC11346858
- DOI: 10.14309/ctg.0000000000000744
Cilofexor in Patients With Compensated Cirrhosis Due to Primary Sclerosing Cholangitis: An Open-Label Phase 1B Study
Abstract
Introduction: This proof-of-concept, open-label phase 1b study evaluated the safety and efficacy of cilofexor, a potent selective farnesoid X receptor agonist, in patients with compensated cirrhosis due to primary sclerosing cholangitis.
Methods: Escalating doses of cilofexor (30 mg [weeks 1-4], 60 mg [weeks 5-8], 100 mg [weeks 9-12]) were administered orally once daily over 12 weeks. The primary endpoint was safety. Exploratory measures included cholestasis and fibrosis markers and pharmacodynamic biomarkers of bile acid homeostasis.
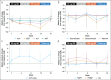
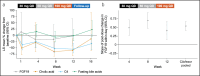
Results: Eleven patients were enrolled (median age: 48 years; 55% men). The most common treatment-emergent adverse events (TEAEs) were pruritus (8/11 [72.7%]), fatigue, headache, nausea, and upper respiratory tract infection (2/11 [18.2%] each). Seven patients experienced a pruritus TEAE (one grade 3) considered drug-related. One patient temporarily discontinued cilofexor owing to peripheral edema. There were no deaths, serious TEAEs, or TEAEs leading to permanent discontinuation. Median changes (interquartile ranges) from baseline to week 12 (predose, fasting) were -24.8% (-35.7 to -7.4) for alanine transaminase, -13.0% (-21.9 to -8.6) for alkaline phosphatase, -43.5% (-52.1 to -30.8) for γ-glutamyl transferase, -12.7% (-25.0 to 0.0) for total bilirubin, and -21.2% (-40.0 to 0.0) for direct bilirubin. Least-squares mean percentage change (95% confidence interval) from baseline to week 12 at trough was -55.3% (-70.8 to -31.6) for C4 and -60.5% (-81.8 to -14.2) for cholic acid. Fasting fibroblast growth factor 19 levels transiently increased after cilofexor administration.
Discussion: Escalating doses of cilofexor over 12 weeks were well tolerated and improved cholestasis markers in patients with compensated cirrhosis due to primary sclerosing cholangitis (NCT04060147).
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Karlsen TH, Folseraas T, Thorburn D, et al. . Primary sclerosing cholangitis: A comprehensive review. J Hepatol 2017;67(6):1298–323. - PubMed
-
- Benito dVM, Rahman M, Lindkvist B, et al. . Factors that reduce health-related quality of life in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2012;10(7):769–75.e2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

