Complications and risk factors on midline catheters' follow-up about non-ICU patients: study protocol for a multicentre observational study (the midDATA study)
- PMID: 37463802
- PMCID: PMC10357646
- DOI: 10.1136/bmjopen-2022-067796
Complications and risk factors on midline catheters' follow-up about non-ICU patients: study protocol for a multicentre observational study (the midDATA study)
Abstract
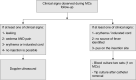
Introduction: The midline catheter (MC) is an increasingly popular device used commonly for patients with difficult venous access or those who require infusion for more than 6 days. Little is known about complications such as infection, thrombosis or occlusion for inpatient and home care patient. This protocol presents the follow-up of non-intensive care unit patients with an MC. The aim is to identify complications and search for risk factors associated with these complications.
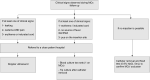
Method and analysis: A prospective observational design is used for the follow-up of 2000 patients from 13 centres in France. Each practitioner (inserting clinician, anaesthetist nurse, hospital nurse and home nurse) will fill out a logbook to report each care made (eg, number of saline flushes, dress maintenance) on the MC and if any complications occurred. The incidence of complications (ie, infections, thrombosis or occlusions) will be expressed by the total number of events per 1000 catheter days. The period of recruitment began in December 2019 for a duration of 2 years. An extension of the inclusion period of 1 year was obtained.
Ethics and dissemination: This study received the approval of the Committee for the Protection of Persons of Nord Ouest IV (No EudraCT/ID-RCB : 2019-A02406-51). It was registered at clinical trials (NCT04131088). It is planned to communicate results at conferences and in a peer-reviewed journal.
Trial registration number: NCT04131088.
Keywords: ANAESTHETICS; Epidemiology; PUBLIC HEALTH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical