Anti-GM-CSF otilimab versus sarilumab or placebo in patients with rheumatoid arthritis and inadequate response to targeted therapies: a phase III randomised trial (contRAst 3)
- PMID: 37696589
- PMCID: PMC10646837
- DOI: 10.1136/ard-2023-224449
Anti-GM-CSF otilimab versus sarilumab or placebo in patients with rheumatoid arthritis and inadequate response to targeted therapies: a phase III randomised trial (contRAst 3)
Abstract
Objectives: To investigate the efficacy and safety of otilimab, an anti-granulocyte-macrophage colony-stimulating factor antibody, in patients with active rheumatoid arthritis and an inadequate response to conventional synthetic (cs) and biologic disease-modifying antirheumatic drugs (DMARDs) and/or Janus kinase inhibitors.
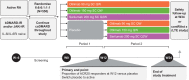
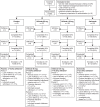
Methods: ContRAst 3 was a 24-week, phase III, multicentre, randomised controlled trial. Patients received subcutaneous otilimab (90/150 mg once weekly), subcutaneous sarilumab (200 mg every 2 weeks) or placebo for 12 weeks, in addition to csDMARDs. Patients receiving placebo were switched to active interventions at week 12 and treatment continued to week 24. The primary end point was the proportion of patients achieving an American College of Rheumatology ≥20% response (ACR20) at week 12.
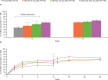
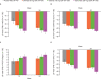
Results: Overall, 549 patients received treatment. At week 12, there was no significant difference in the proportion of ACR20 responders with otilimab 90 mg and 150 mg versus placebo (45% (p=0.2868) and 51% (p=0.0596) vs 38%, respectively). There were no significant differences in Clinical Disease Activity Index, Health Assessment Questionnaire-Disability Index, pain Visual Analogue Scale or Functional Assessment of Chronic Illness Therapy-Fatigue scores with otilimab versus placebo at week 12. Sarilumab demonstrated superiority to otilimab in ACR20 response and secondary end points. The incidence of adverse or serious adverse events was similar across treatment groups.
Conclusions: Otilimab demonstrated an acceptable safety profile but failed to achieve the primary end point of ACR20 and improve secondary end points versus placebo or demonstrate non-inferiority to sarilumab in this patient population.
Trial registration number: NCT04134728.
Keywords: autoimmune diseases; biological therapy; cytokines; inflammation; rheumatoid arthritis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PCT has received consulting fees from AbbVie, Biogen, Bristol Myers Squibb, Fresenius, Galapagos, Gilead Sciences, GSK, Janssen, Lilly, Nordic Pharma, Pfizer, Roche, Sanofi and UCB, and research support from Galapagos. MEW receives research support from AbbVie, Aqtual, Bristol Myers Squibb and Lilly, and consultation fees from AbbVie, Aclaris, Amgen, Bayer, Bristol Myers Squibb, Corvitas, Genosco, Gilead Sciences, GSK, Horizon, Johnson & Johnson, Lilly, Novartis, Pfizer, Rami Therapeutics, R Pharma, Roche, Sanofi, Scipher, Sci Rhom, Set Point and Tremeau. He holds stock/stock options of CanFite, Inmedix and Scipher. IBMcI has received consultancy and research support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Causeway, Compugen, Gilead Sciences, GSK, Lilly, Novartis, Pfizer and UCB and holds a leadership role in Evelo, University of Glasgow, Versus Arthritis and is an NHS GGC Board Member and an Annals of the Rheumatic Diseases Editorial Board Member. TA has accepted research grants and/or honoraria for meetings from AbbVie, Alexion, Astellas Pharma, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, GSK, Lilly Japan, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Takeda Pharmaceutical and UCB Japan. VS has received consulting fees from AbbVie, Alpine, Alumis, Amgen, Aria, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, RAPT, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, Tonix and Urica. TT received payment or honoraria from AbbVie, Asahi Kasei, Astellas, AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, Janssen, Lilly Japan, Mitsubishi-Tanabe, Pfizer Japan and is an Annals of the Rheumatic Diseases Editorial Board Member. MB, DB, JD, CG, AG, SM, CO’S, DS, LAS, CS, JES, MW, RW and SW are employees of GSK and hold GSK stock/shares. RMF has received research support from AbbVie, Amgen, Arthrosi, AstraZeneca, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Galvani, Genentech/Roche, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung, Sanofi-Genzyme, Selecta and UCB; consulting fees from AbbVie, Amgen, Arthrosi, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Galapagos, Galvani, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung and UCB and honoraria from AbbVie, GSK and Pfizer and is an Annals of the Rheumatic Diseases Editorial Board Member.
Figures