The effect of probiotics on functional constipation in adults: A randomized, double-blind controlled trial
- PMID: 36316826
- PMCID: PMC9622669
- DOI: 10.1097/MD.0000000000031185
The effect of probiotics on functional constipation in adults: A randomized, double-blind controlled trial
Abstract
Background: Two formulations were developed in the form of an oral sachet containing probiotics, and their efficacy and safety were evaluated in adults with functional constipation.
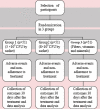
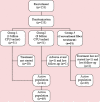
Methods: One formulation with Lactobacillus acidophilus, Bifidobacterium bifidum and Lactobacillus rhamnosus (3 billion Colony Forming Units - CFU); and another with Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium longum, Bifidobacterium lactis, Lactobacillus casei, Bifidobacterium animallis (8 billion CFU). The participants were randomized in a 3-arm parallel study and one oral sachet was auto-administered once a day for 30 days.
Results: Primary outcomes were improvement in increasing the frequency of weekly bowel movements and improvement in stool quality. Secondary outcomes were number of adverse events. In the first week one observed an increase in stool frequency and in the quality of stools, showing an improvement in constipation. No statistically significant differences were observed between the three treatment groups in relation to these outcomes (P ≥ .05). Only one adverse event was observed in a patient of group 2, related to abdominal pain.
Conclusion: The two probiotic cocktails were effective in improving the symptoms of functional constipation, by increasing both the weekly frequency of evacuation and stool quality, and were deemed safe. Clinicaltrials.gov number: NCT04437147.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Galvão-Alves J. Constipação intestinal. J Bras Med. 2013;101:31–7.
-
- Sobrado CW, Neto IJFC, Pinto RA, et al. . Diagnosis and treatment of constipation: a clinical update based on the Rome IV criteria. J Coloproctol. 2018;38:137–44.
-
- Lewis SJ, Heaton KWJ. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical