Determination of Urinary Gluten Immunogenic Peptides to Assess Adherence to the Gluten-Free Diet: A Randomized, Double-Blind, Controlled Study
- PMID: 34613954
- PMCID: PMC8500619
- DOI: 10.14309/ctg.0000000000000411
Determination of Urinary Gluten Immunogenic Peptides to Assess Adherence to the Gluten-Free Diet: A Randomized, Double-Blind, Controlled Study
Abstract
Introduction: The adherence to a gluten-free diet (GFD) is a trending topic in the management of celiac disease. The aim of our study was to evaluate the diagnostic performance of urinary gluten immunogenic peptides (GIP) determination to detect gluten contamination of the GFD.
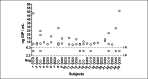
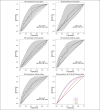
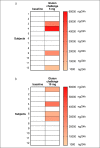
Methods: In study A, 25 healthy adults on a standard GFD performed 6 gluten challenges (0, 10, 50, 100, 500, and 1,000 mg) with quantification of urinary GIP before (T0) and during the following 24 hours. In study B, 12 participants on a gluten contamination elimination diet underwent urinary GIP determination at T0 and after challenge with 5 or 10 mg gluten. Urine GIP concentration was determined by an immunochromatographic assay.
Results: In study A, 51 of 150 baseline urine samples were GIP+ on GFD and 7 of 17 were GIP+ after the zero-gluten challenge, whereas only 55 of 81 were GIP+ after the 10-1,000 mg gluten challenges. There was no significant change in the 24-hour urinary GIP when increasing gluten from 10 to 1,000 mg. In study B, 24 of 24 baseline urine samples were GIP-, whereas 8 of 24 were GIP+ after 5 or 10 mg of gluten.
Discussion: Traces of gluten in the standard GFD may cause positivity of urinary GIP determination, whereas a false negativity is common after a gluten intake of 10-1,000 mg. Owing to the impossibility of standardizing the test in normal conditions, it seems unlikely that urinary GIP determination may represent a reliable tool to assess the compliance to the GFD of patients with celiac disease or other gluten-related disorders.
Trial registration: ClinicalTrials.gov NCT04477239.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures
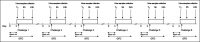


Comment in
-
Controls and Result Interpretations in Studies of Urine Gluten Peptide Determinations.Clin Transl Gastroenterol. 2022 Jan 19;13(1):e00456. doi: 10.14309/ctg.0000000000000456. Clin Transl Gastroenterol. 2022. PMID: 35060931 Free PMC article. No abstract available.
-
Urinary Gluten Peptide Determination: Results Are Results, Even When They Contradict Aprioristic Expectations.Clin Transl Gastroenterol. 2022 Apr 1;13(4):e00472. doi: 10.14309/ctg.0000000000000472. Clin Transl Gastroenterol. 2022. PMID: 35297394 Free PMC article. No abstract available.
References
-
- Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology 2001;120:636–51. - PubMed
-
- Singh P, Arora A, Strand TA, et al. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:823–36 e2. - PubMed
-
- Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol 2011;30:219–31. - PubMed
-
- Lee HJ, Anderson Z, Ryu D. Gluten contamination in foods labeled as "gluten free" in the United States. J Food Prot 2014;77:1830–3. - PubMed
-
- Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007;85:160–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

