Fosciclopirox suppresses growth of high-grade urothelial cancer by targeting the γ-secretase complex
- PMID: 34059639
- PMCID: PMC8166826
- DOI: 10.1038/s41419-021-03836-z
Fosciclopirox suppresses growth of high-grade urothelial cancer by targeting the γ-secretase complex
Abstract
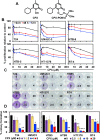
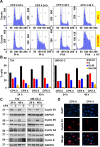
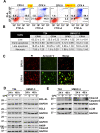
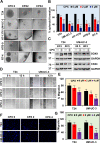
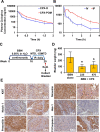
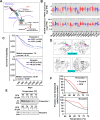
Ciclopirox (CPX) is an FDA-approved topical antifungal agent that has demonstrated preclinical anticancer activity in a number of solid and hematologic malignancies. Its clinical utility as an oral anticancer agent, however, is limited by poor oral bioavailability and gastrointestinal toxicity. Fosciclopirox, the phosphoryloxymethyl ester of CPX (Ciclopirox Prodrug, CPX-POM), selectively delivers the active metabolite, CPX, to the entire urinary tract following parenteral administration. We characterized the activity of CPX-POM and its major metabolites in in vitro and in vivo preclinical models of high-grade urothelial cancer. CPX inhibited cell proliferation, clonogenicity and spheroid formation, and increased cell cycle arrest at S and G0/G1 phases. Mechanistically, CPX suppressed activation of Notch signaling. Molecular modeling and cellular thermal shift assays demonstrated CPX binding to γ-secretase complex proteins Presenilin 1 and Nicastrin, which are essential for Notch activation. To establish in vivo preclinical proof of principle, we tested fosciclopirox in the validated N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) mouse bladder cancer model. Once-daily intraperitoneal administration of CPX-POM for four weeks at doses of 235 mg/kg and 470 mg/kg significantly decreased bladder weight, a surrogate for tumor volume, and resulted in a migration to lower stage tumors in CPX-POM treated animals. This was coupled with a reduction in the proliferation index. Additionally, there was a reduction in Presenilin 1 and Hes-1 expression in the bladder tissues of CPX-POM treated animals. Following the completion of the first-in-human Phase 1 trial (NCT03348514), the pharmacologic activity of fosciclopirox is currently being characterized in a Phase 1 expansion cohort study of muscle-invasive bladder cancer patients scheduled for cystectomy (NCT04608045) as well as a Phase 2 trial of newly diagnosed and recurrent urothelial cancer patients scheduled for transurethral resection of bladder tumors (NCT04525131).
Conflict of interest statement
M.T. and S.J.W. are co-inventors of fosciclopirox composition of matter patents issued in the US, Europe, and Japan as well as a methods of use patents issued in the US. S.A. and S.J.W. are inventors on an issued patent in Europe, and a pending application in China. The exclusive, non-terminable rights to intellectual property generated by the inventors, as employees of the University, were licensed by the University of Kansas to CicloMed LLC in 2015. CicloMed is developing fosciclopirox for the treatment of bladder cancer. Kansas Life Sciences Development Company, a subsidiary of the University of Kansas Medical Center Research Institute, Inc. (which is a private, not-for-profit 501(c)(3) corporation established to promote and support medical research and faculty invention disclosures), possesses a financial interest in CicloMed.
Figures

References
-
- NCI. in Reports on Cancer: Annual Report to the Nation (NCI, 2018).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

