The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial
- PMID: 36742008
- PMCID: PMC9889936
- DOI: 10.3389/fnut.2022.1023997
The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial
Abstract
Background: Curcumin (CUR) and quercetin (QUE), two natural polyphenols, possess diverse biological activities including broad-spectrum antiviral, antioxidant, and immunomodulatory effects. Both CUR and QUE have shown inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in in vitro assays.
Objective: In the present study we aimed to assess the possible treatment benefits of a combined curcumin and quercetin (CUR-QUE) oral supplement, alongside standard of care (SOC), in the early-stage COVID-19 infection.
Methods: This was an exploratory, pragmatic, open-label, randomized controlled clinical trial, conducted at the Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, PK. The study compared the treatment effect of an oral CUR-QUE supplement plus SOC vs. SOC alone, in the early-stage/mild to moderately symptomatic COVID-19 outpatients. Patients were randomized in a 1:1 ratio to CUR-QUE (n = 25) and control (n = 25) treatment groups. The CUR-QUE supplementation consisted of a daily intake of 168 mg curcumin and 260 mg quercetin, as two soft capsules, to be taken twice a day at home for 14 days.
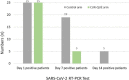
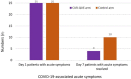
Results: After one-week of treatment, most of the patients in the CUR-QUE group showed an expedited clearance of the viral infection i.e., 18 (72.0%) vs. 6 (24.0%) patients in the control group tested negative for SARS-CoV-2 in the nasal-oropharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) analysis (p = 0.0002). In addition, COVID-19-associated acute symptoms were also speedily resolved in the CUR-QUE treated patients, i.e., 10 (40.0%) vs. 4 (16.0%) patients in the control group (p = 0.061). The CUR-QUE supplementation therapy was well-tolerated by all 25 patients and no treatment-emergent effects or serious adverse events were reported.
Conclusion: The results revealed in this exploratory study suggest a possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19. It is proposed that the two agents possibly acting in synergy, interfere the SARS-CoV-2 replication, and thus help a speedy recovery in the early-stage of COVID-19. Further research is highly encouraged.
Clinical trial registration: Clinicaltrials.gov, Identifier NCT04603690.
Keywords: COVID-19; SARS-CoV-2; curcumin; polyphenols; quercetin.
Copyright © 2023 Ujjan, Khan, Nigar, Ahmed, Ahmad and Khan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

